What exactly is the coronavirus vaccine? Tests, hidden components and the truth about the timing of the development of Sputnik V
February 5, 2021
The technology that formed the basis for the development of the Sputnik V vaccine has long been known to science. The principle of its operation is simple: the adenovirus, deprived of the ability to reproduce, plays the role of delivering a piece of the SARS-CoV-2 gene. The adenoviral vector was first used 40 years ago to create a vaccine against Ebola.
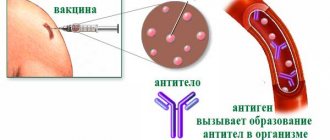
Institute named after Gamaleya, where Sputnik V was synthesized, has been creating vaccines based on adenovirus since 2015, so scientists managed to create a vaccine against coronavirus in a relatively short time. It protects the body from infection in the following way: a gene encoding the S-protein of the coronavirus spike was inserted into an adenoviral vector (virus), which was artificially deprived of the ability to reproduce. The vector delivers a piece of RNA virus into the cell, and the cell, in turn, reacts to it in the same way as it would react to an invasion by SARS-CoV-2. Specific antibodies begin to be produced, which create immunity against coronavirus.
What is included in the coronavirus vaccine and does it contain dangerous “ingredients”?
The adenoviral vector is the main active ingredient of the vaccine. This is the main component, but other components are also necessary for the vaccination to work. They help maintain the effect of the active substance and facilitate its penetration into human cells.
In addition to the adenoviral vector itself, Sputnik V includes:
- Tris is a compound that maintains the acidity (pH) necessary for the vaccine to work.
- Sodium chloride (or saline) - the drug is diluted with it to increase its availability to cells.
- Magnesium chloride hexahydrate is added to the vaccine to restore and maintain the amount of water in the extracellular and intracellular space. This is necessary to prevent cell dehydration.
- EDTA (ethylenediaminetetraacetic acid) is used to preserve the genetic piece of SARS-CoV-2 embedded in the adenoviral vector, since the RNA is unstable outside the virus.
- Polysorbate – binds vaccine components.
- Sucrose, ethanol and water - for the sterility of the vaccine and its better “uptake” by cells
Every “ingredient” of the coronavirus vaccine is absolutely harmless. In order to check whether all these components together form a stable immunity to coronavirus, tests are being carried out.
What tests has the coronavirus vaccine undergone?
Immediately after synthesizing the new drug, scientists conducted preclinical testing on hamsters and primates. The toxicity, safety and effectiveness of the new vaccine were tested. There were no complications or adverse reactions in animals, so scientists began clinical trials on humans.
Phase 1 trials are usually conducted on a small group of healthy volunteers. To test the Russian Sputnik V vaccine, 76 military personnel aged from 18 to 60 years were recruited. The phase was divided into two stages.
The first stage lasted 28 days. Nineteen volunteers were given the first part of the vaccine, based on a weakened adenovirus type 26, and the other nineteen were given the second part, with adenovirus type 5. This way the safety of both components of the vaccine was tested. The result of this stage was good tolerability of the drug by all volunteers. Side effects were mild to moderate:
- pain at the injection site (58%);
- increase in temperature (50%);
- headache (42%);
- weakness (28)%;
- pain in muscles and joints (24%);
No life-threatening side effects were found.
During the second stage, immunity already developing after vaccination was studied. All volunteers received both parts of the vaccine with a 21-day gap between them. On day 28 after the second dose, using high-precision tests, antibodies to SARS-CoV-2 were detected in 100% of volunteers. Moreover, the formation of another – cellular immunity – has been proven. Usually it remains in the body’s “memory” for several years.
Three months after the start of the third phase of clinical trials in December 2021, the Russian Ministry of Health decided to combine the last stage of research with mass vaccination of the population with the Sputnik V vaccine. The decision was made quickly, based on preliminary results published by the vaccine developers in November 2021.
The calculations are based on cases of COVID-19 among already vaccinated volunteers. Of the total 18,794 trial participants who received both vaccine injections or a placebo, 39 people were diagnosed with the virus by the end of the third week after the second shot.
31 cases of infection were identified in those who received the placebo. In the group of volunteers vaccinated with the real vaccine, COVID-19 was diagnosed in eight (the group who received the vaccine was three times more likely than the placebo group). Comparison of the results for the two groups gives the vaccine effectiveness at 95%.
“The recruited group of study participants allows us to fully study the safety profile and preventive effectiveness of the vaccine over a long period of time,” the Ministry of Health explained.
Despite the fact that the Sputnik V vaccine became available to everyone (with the exception of the group with contraindications), scientists continued to monitor the volunteers who took part in the third phase of clinical trials. February 2, 2021 The vaccine developers presented the results of the study in the authoritative medical publication The Lancet.
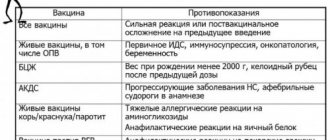
Contraindications
Only a doctor can decide whether Polimilex is suitable for vaccination
"Polimilex" is contraindicated if there is a history of an allergic reaction to any component of the vaccine.
Vaccination "Polimilex" is contraindicated in the following cases:
- Severe reaction to a previous vaccine.
- Known hypersensitivity to one or more components of the vaccine.
- A disease accompanied by fever, an acute infectious or chronic disease in the acute stage. Vaccination is carried out 2-4 weeks after recovery or during the period of convalescence or remission. For mild ARVI, acute intestinal diseases, etc., vaccinations are carried out immediately after the temperature has normalized.
- Severe reaction (temperature above 40 °C, swelling and hyperemia at the injection site greater than 8 cm in diameter) or a complication of a previous drug administration.
Results of the latest third phase of clinical trials of the Sputnik V vaccine
The third phase of testing of the Sputnik V vaccine lasted from September 7 to November 24. 21,977 people took part in it, of which 16,427 were vaccinated, and 5,435 received a placebo (a solution that contains all the ingredients of Sputnik V, except for the adenoviral vector itself).
According to the scientists’ findings, specific antibodies against coronavirus were developed 42 days after the first vaccination or 21 days after the second in 95.8% of volunteers. Moreover, on the day of the first injection and 28 days after the second, cellular immunity was already determined in the volunteers.
As for side effects, they occurred in 7966 vaccinated people, while in 94% of them they were mild and included fever, discomfort at the injection site, headache and weakness. Only 0.3% of those vaccinated had serious side effects, however, an independent commission did not prove a connection between them and vaccination.
The effectiveness of the vaccine at these rates is 91.6%.
Sputnik V was also studied on volunteers over 60 years of age - a total of 2,144 people. The effectiveness of the vaccine in this group is practically no different from the general one and is 91.8%.
Thus, the vaccine has shown its effectiveness and safety both in people from 18 to 60 years of age and in elderly volunteers. However, no serious side effects associated with the vaccine were found.
Biological and immunobiological properties The vaccine creates long-term immunity to polio virus types 1, 2, 3 in the majority of vaccinated people (90-95%).
Purpose: Active prevention of polio. Children aged 3 months to 14 years are subject to routine vaccinations.
Method of administration The vaccine is intended for oral use. Under no circumstances should this vaccine be administered parenterally.
Vaccinations with oral polio vaccine are carried out 6 times, at the age established by the preventive vaccination calendar. Age: 3 months 4.5 months 6 months 18 months 20 months 14 years
The first three vaccinations make up the vaccination course. Extending the intervals between vaccinations is allowed in exceptional cases; in the presence of medical contraindications, shortening the intervals between the first three vaccinations is not allowed. It is allowed to reduce the interval between the third and fourth vaccinations to 3 months, if the intervals between the first three vaccinations have been significantly extended. Children arriving in this territory without proof of vaccinations are subject to three-time immunization. Subsequent vaccinations of these children are carried out in accordance with age.
Vaccinations according to epidemiological indications are carried out when polio diseases occur in a children's institution or locality. The number of persons subject to immunization according to epidemiological indications and the frequency of vaccinations are established in each specific case, taking into account the characteristics of the polio epidemic process. Subsequently, regular scheduled vaccinations against polio are carried out at the prescribed time.
Vaccinations against polio are allowed to be carried out on the same day as vaccination with DTP vaccine (ADS or ADS-M toxoid); simultaneous administration of polio vaccine with other drugs in the vaccination calendar is allowed.
The vaccine is used 4 or 2 drops per dose in accordance with the packaging of the drug. The vaccination dose of the vaccine is instilled into the mouth with a dropper or pipette attached to the bottle 1 hour before meals. It is not allowed to take the vaccine with water or any other liquid, or to eat or drink within an hour after vaccination.
Unused vaccine from an opened vial can be stored for no more than 2 days at a temperature of (6 ± 2) ° C in a vial tightly closed with a dropper or rubber stopper. If necessary, it is allowed to draw the vaccine with a sterile syringe through a rubber stopper, subject to aseptic rules. In this case, the vaccine remaining in the vial can be used until the expiration date.
Reaction to vaccine administration There is practically no reaction to vaccine administration. In some vaccinated people who are predisposed to allergic reactions, allergic complications in the form of a rash such as urticaria or Quincke's edema can extremely rarely be observed. Vaccine-associated diseases, which are observed no more often than 1 case per 3 million vaccinated children, are extremely rare both in vaccinated people and in persons in contact with vaccinated people. They always require a differential diagnosis with polio-like diseases. To limit the circulation of the vaccine virus among those around the vaccinated child, parents should be explained the need to observe the rules of personal hygiene of the child after vaccination (separate bed, potty, bedding, clothes separate from other children, etc.).
Contraindications Live polio vaccine is a safe and areactogenic drug. Contraindications are:
- Neurological disorders accompanying previous vaccination with polio vaccine;
- Immunodeficiency state (primary), malignant neoplasms, immunosuppression (vaccinations are carried out no earlier than 6 months after the end of the course of therapy);
- Routine vaccination is postponed until the end of acute manifestations of the disease and exacerbation of chronic diseases. For mild ARVI, acute intestinal diseases, etc., vaccinations are carried out immediately after the temperature normalizes.
Storage conditions Store frozen at minus (20±1)°C in special low-temperature refrigerators or in liquid form at (6±2)°C. It is permissible to defrost and re-freeze the vaccine, but no more than three times throughout the entire storage period. Shelf life : At minus 20°C – two years, at temp. (6±2)°C - 6 months.
Fast does not mean bad: why was the coronavirus vaccine developed in a short time?
The development and testing of the vaccine took about a year. This is a unique, but understandable, case. First of all, the creation of Sputnik V was accelerated by the fact that the vaccine was made on the basis of an adenoviral vector. This technology has long been known to scientists, and the center itself. Gamaleyi had experience in producing similar vaccines. A vaccine against Middle East respiratory syndrome was previously developed. The virus that causes it, MERS, also belongs to the group of coronaviruses.
The rapid spread and ability of the virus to mutate required drastic decisions. At the same time, the safety and effectiveness of the vaccine comes first. That is why the first phases of the study were carried out in compliance with all international rules: the number of volunteers in each phase, the criteria for assessing their health status after vaccination. Only after the vaccine showed its safety and ability to protect against coronavirus was the decision made on mass vaccination.