Compatibility with other vaccines
Vaccination against rubella can be carried out simultaneously (on the same day) with other calendar vaccinations (against whooping cough, diphtheria, tetanus, mumps, measles, polio, hepatitis B) or no earlier than 1 month after the previous vaccination. When simultaneous vaccination, drugs are administered to different places; mixing vaccines in one syringe is prohibited.
After the administration of human blood products (immunoglobulin, plasma, etc.), the vaccine is administered no earlier than 3 months later. After administration of the rubella vaccine, blood products can be administered no earlier than 2 weeks later. If it is necessary to use immunoglobulin earlier than this period, vaccination against rubella should be repeated after 3 months.
If there are antibodies to the rubella virus in the blood serum, repeated vaccination is not carried out. Tuberculin tests are recommended to be performed before or 4-6 weeks after administration of the rubella vaccine. After the prescription of immunosuppressants and radiation therapy, vaccination is carried out no earlier than 12 months after the end of treatment.
Rubella vaccine
Cultural live attenuated rubella vaccine, lyophilisate for preparing a solution for subcutaneous administration, made from “Live attenuated rubella vaccine, substance – frozen solution.” Purpose
Prevention of rubella. Routine vaccinations are carried out twice at the age of 12 months and 6 years. A single vaccination for girls aged 13 years who have not previously been vaccinated and have not had rubella, or girls who have received only one vaccination. Vaccination against rubella of persons who have not been sick and not previously vaccinated is carried out in accordance with the National Calendar of Preventive Vaccinations of the Russian Federation: children from 5 to 17 years old, girls from 18 to 25 years old. Vaccinations may also be given to other groups of the population.
Contraindications
- immunodeficiency states; malignant blood diseases and neoplasms. When prescribing immunosuppressants and radiation therapy, vaccination is carried out no earlier than 12 months after the end of treatment;
- pregnancy;
- severe reaction (temperature rise above 40°C, swelling, hyperemia more than 8 cm in diameter at the site of vaccine administration) or a complication to the previous dose;
- acute infectious and non-infectious diseases, exacerbation of chronic diseases - vaccination should be carried out no earlier than 1 month after recovery.
Note: HIV infection is not a contraindication to vaccination.
Warnings
The vaccine should not be administered during pregnancy. Precautions must be taken to avoid conception for 2 months after vaccination.
Interaction with other drugs
Vaccination against rubella can be carried out simultaneously (on the same day) with other calendar vaccinations (against whooping cough, diphtheria, tetanus, mumps, measles, polio, hepatitis B) or no earlier than 1 month after the previous vaccination. When simultaneous vaccination, drugs are administered to different places; mixing vaccines in one syringe is prohibited.
Immunoglobulins and blood products:
After administration of blood products (immunoglobulin, plasma, etc.), the vaccine is recommended to be administered no earlier than 3 months later. After administration of the rubella vaccine, blood products can be administered no earlier than 2 weeks later; If it is necessary to use immunoglobulin earlier than this period, vaccination against rubella should be repeated after 3 months. If there are antibodies to the rubella virus in the blood serum, repeated vaccination is not carried out.
Directions for use and dosage
Immediately before use, the vaccine is diluted with a solvent (water for injection) at the rate of 0.5 ml of solvent per one vaccination dose of the vaccine. To avoid foaming, the vaccine is dissolved by gently shaking the ampoule. The vaccine should completely dissolve within 3 minutes. The dissolved vaccine is a clear liquid from light yellow to pink. The vaccine and solvent in ampoules with damaged integrity, labeling, or changes in their physical properties (color, transparency, etc.) or improperly stored are not suitable for use.
The opening of ampoules and the vaccination procedure are carried out in strict compliance with the rules of asepsis and antiseptics. The ampoules at the incision site are treated with 70° alcohol and broken off, while preventing alcohol from getting into the ampoule. To dilute the vaccine, suck out the entire required volume of solvent and transfer it to an ampoule with dry vaccine. After mixing, the vaccine is drawn into a sterile syringe with another needle and used for vaccination.
The vaccine is administered subcutaneously in a dose of 0.5 ml into the shoulder area after pre-treating the skin at the site of vaccine administration with 70° alcohol.
The dissolved vaccine is used immediately and cannot be stored.
The vaccination performed is registered in the established registration forms, indicating the name of the drug, date of vaccination, dose, manufacturer, batch number, expiration date, reaction to the vaccination.
Side effects
At the injection site, short-term hyperemia, swelling and induration may develop, accompanied by pain. The vaccine may cause the following adverse reactions of varying severity in some vaccinated individuals:
- rash;
- short-term increase in temperature to subfebrile levels; higher temperature in some vaccinated people;
- cough, runny nose, malaise, headache;
- nausea;
- lymphadenopathy (enlargement of predominantly occipital and posterior cervical lymph nodes).
Those vaccinated in post-pubertal age may experience arthralgia or arthritis, and in rare cases, polyneuritis.
All these reactions are characterized by a short-term course and go away without treatment.
Contraindications
Only a doctor can decide whether Rubella is suitable for vaccination
The Rubella vaccine is contraindicated if there is a history of an allergic reaction to any component of the vaccine, as well as in the following cases:
- Allergic reactions to vaccine components.
- Acute infectious and non-infectious diseases, exacerbation of chronic diseases.
- Immunodeficiency conditions; malignant blood diseases and neoplasms.
- Pregnancy and breastfeeding period.
- A severe reaction (temperature rise above 40 C, swelling, hyperemia more than 8 cm in diameter at the site of vaccine administration) or a complication to the previous dose of the vaccine.
Information about the disease
Rubella is caused by a highly contagious virus. It spreads through airborne droplets and household contact: with saliva when talking, coughing and sneezing, as well as through shared utensils and toys. At risk for infection:
- large families;
- health workers;
- kindergarten and school workers;
- unvaccinated people who have not had rubella;
- people with weakened immune systems - with low levels of protective antibodies.
The asymptomatic period is 10–21 days. The disease begins with an increase in temperature to 38-39 °C. Hyperthermia is accompanied by:
- general weakness;
- headache;
- lacrimation;
- photophobia;
- runny nose;
- sore throat and cough;
- swollen lymph nodes;
- rash.
The diagnosis is made based on the results of general and enzyme-linked immunosorbent blood tests. Rubella is treated by an infectious disease specialist. For complications: encephalitis, nephritis, arthritis, bacterial infections, exacerbation of chronic pathologies, hospitalization is indicated.
Only symptomatic treatment is available to medicine; there is no specific therapy for rubella. Therefore, it is extremely important for children to be vaccinated in accordance with the national calendar - at 1 year and at 6 years. In addition, administering the vaccine to teenage girls and women of childbearing age planning to have children, aged 18–25 or more, is effective.
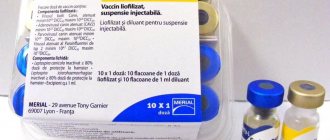
M-M-R II Vaccine against measles, mumps and rubella, live lyof. 1 dose vial in a set with a solution for subcutaneous application
Method of administration and dosage FOR SUBSCUTANEOUS ADMINISTRATION. THE VACCINE CANNOT BE ADMINISTERED INTRAVENOUSLY. Human immunoglobulin should not be administered simultaneously with the vaccine (see section “interaction with other drugs”),
The vaccine is administered subcutaneously, preferably into the outer surface of the upper third of the shoulder. The vaccine dose is the same for all ages and is 0.5 ml.
Cautions:
For each injection and/or dissolution of the vaccine, you should use a sterile syringe that does not contain preservatives, antiseptics and detergents, because these substances can inactivate the live vaccine virus.
To dissolve the vaccine, use only the sterile solvent supplied with the vaccine (water for injection), because it does not contain preservatives or other antiviral substances that could inactivate the vaccine.
Each bottle of reconstituted solution of the M-M-P II ® vaccine must be visually inspected for the absence of mechanical particles and color changes before injection. The reconstituted solution of M-M-P II ® vaccine should be clear and yellow in color.
Recommended vaccination schedule:
Children first vaccinated at 12 months of age and older should be revaccinated according to the national vaccination schedule at 6 years of age.
Other aspects of vaccination:
Women of childbearing age.
Immunization of non-pregnant, non-immune girls and women of childbearing age with a live attenuated vaccine against rubella, measles and mumps is indicated subject to certain precautions (see section “use during pregnancy and 13 breastfeeding period”). Vaccination of non-immune women of childbearing age protects them from rubella during pregnancy, which in turn prevents the fetus from becoming infected and developing congenital rubella lesions.
Women of childbearing age are advised to protect themselves from pregnancy for 3 months after vaccination. They should be informed of the reasons for such precautions (see section “Use during pregnancy and breastfeeding”).
Conducting serological studies of women of childbearing age to determine their susceptibility to rubella, followed by vaccination of seronegative individuals, is desirable, but not mandatory.
Women of childbearing age should be informed of the high likelihood of developing usually transient arthralgia or arthritis 2-4 weeks after vaccination (see section "Side Effects").
Women in the postpartum period:
In many cases, vaccination of women susceptible to rubella immediately after childbirth is justified (see section “use during pregnancy and breastfeeding”).
Other settlement groups:
Unvaccinated and rubella-naïve children over 12 months of age who are in contact with a susceptible pregnant woman should be vaccinated against rubella (monovalent rubella vaccine or M-M-P II ® vaccine) to reduce the risk of possible infection in the pregnant woman.
Vaccination is also recommended for susceptible individuals from high-risk groups, such as students, health care workers, and military personnel.
Non-immune persons may become infected with measles, mumps and rubella viruses during their stay abroad and bring them to their country of permanent residence. Before travel, persons susceptible to one or more of these diseases can be given either the monovalent vaccine or the M-M-P II* vaccine. Persons susceptible to mumps and rubella viruses are recommended to receive the M-M-P II vaccine; persons susceptible to the measles virus in the absence of a monovalent measles vaccine are recommended to receive the M-M-P II vaccine, regardless of their immune status regarding mumps and rubella viruses.
According to exposure protective vaccination:
Vaccination of contacts of a person with measles may provide some protection if the vaccine is administered within 72 hours of exposure. If the vaccine was administered several days before infection, then in this case a high preventive effect will be achieved. There are no definitive data on the effectiveness of vaccination of contacts of a patient with mumps and rubella.
Use with other vaccines:
The M-M-P II® vaccine should be administered 1 month before or 1 month after the administration of other live viral vaccines.
M-M-P II® vaccine was administered concomitantly with Haemophilus influenza type b conjugate inactivated vaccine and live attenuated varicella vaccine, with the vaccines administered using different syringes at different sites on the body. No disturbances in the immune response to the administered antigens were found, and the nature, frequency and severity of adverse reactions were similar to those when the vaccines were administered separately. The use of DTP (diphtheria-tetanus-pertussis vaccine) and/or OPV (oral polio vaccine) concomitantly with the measles, mumps, and rubella vaccine is not recommended due to limited data on the results of their simultaneous administration.
Other immunization regimens were also used. Data from published studies regarding coadministration of recommended vaccines for routine immunization (eg, DTP [or DTaP], IPV [or OPV], Haemophilus influenzae type b vaccine with or without hepatitis B vaccine, and varicella vaccine) with other pediatric vaccines (live, attenuated or inactivated) have not shown any interaction between them.
Recommendations for preparing and conducting vaccination:
Fill the syringe completely with solvent. Add all the solvent to the vial of lyophilized vaccine and mix thoroughly. Draw the entire contents of the vial into a syringe and inject completely subcutaneously. It is recommended to use the vaccine as soon as possible after dissolution.
To prevent transmission of hepatitis B virus and other infectious agents, disposable, sterile syringes and needles should be used.