Instructions for use of the Rabikan anti-rabies inactivated vaccine
dry culture from the Shchelkovo-51 strain for dogs and cats
(Developer organization: FKP “Shchelkovo Biocombine”, Moscow region)
I. General information
International non-proprietary name - inactivated dry culture anti-rabies vaccine from the Shchelkovo-51 strain for dogs and cats (Rabican).
Dosage form - lyophilized mass.
The vaccine is made from β-propiolactone-inactivated rabies virus strain “Shchelkovo-51” with the addition of 33.3% sucrose-peptone-gelatin stabilizer.
In appearance, the vaccine is a dry, porous mass of yellowish-brown color, which, when sterile distilled water is added in a volume equal to the volume of the vaccine in the vial before drying, dissolves within 1-2 minutes to form a transparent yellowish-brown liquid.
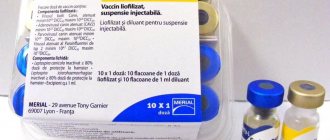
The vaccine is packaged in 2 packs; 4 or 10 cm3 (1; 2; 5 doses for large breed dogs or 2; 4; 10 doses for cats, puppies and adult dogs of small decorative breeds) in bottles of appropriate capacity, hermetically sealed with rubber stoppers reinforced with aluminum caps.
The vaccine vials are packaged in boxes with dividing partitions to ensure their integrity. Each box of vaccine contains instructions for its use. Boxes with the vaccine are packed in boxes or corrugated containers.
The shelf life of the vaccine is 24 months from the date of release, subject to storage and transportation conditions. After the expiration date, the vaccine is not suitable for use.
The vaccine is stored and transported in a dry, dark place at a temperature of 2°C to 8°C or at any sub-zero temperature.
It is allowed to transport the vaccine in the packaging of the manufacturer at a temperature not exceeding 25°C for no more than 10 days.
The Rabican vaccine should be stored out of the reach of children.
Vaccine in vials without markings, with a violation of the integrity and/or tightness of the closure, with a changed color and/or consistency, with the presence of foreign impurities, with an expired expiration date, as well as vaccine residues not used within 4 hours after opening the vials are subject to discarding and disinfection by boiling for 30 minutes or treating with a 2% alkali solution or a 5% chloramine solution (1:1) for 30 minutes. Vaccine vials, syringes and needles are treated in the same way.
Disposal of decontaminated vaccine does not require special precautions.
II. Biological properties
The vaccine causes the formation of an immune response to the rabies pathogen in primary vaccinated animals from 5-7 days after a single injection of the vaccine; immunity reaches its maximum by 30-40 days and lasts for 1 year. In the case of a two-time vaccine administration, with revaccination after 30-50 days, immunity lasts for 2 years.
The immunogenic activity (immunogenicity index) of the vaccine is at least 1 IU/cm3.
The vaccine is harmless and has no medicinal properties.
III. Application procedure
The vaccine is intended for preventive and emergency vaccination of dogs and cats against rabies.
It is prohibited to vaccinate animals suspected of having rabies.
Dogs and cats are subject to vaccination starting from 2 months of age. Vaccination is carried out in compliance with the rules of asepsis and antiseptics.
Before use, the dry vaccine is reconstituted to the original volume indicated on the label with sterile distilled water.
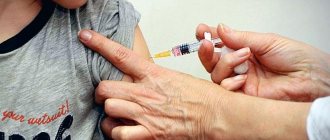
The drug is administered subcutaneously. The injection site is wiped with a 70% solution of ethyl alcohol or another disinfectant solution. A separate needle is used for each animal.
The vaccine is administered in doses (per injection):
- dogs of large breeds (shepherds, St. Bernards, etc.) - 2 cm3;
- puppies (up to 1 year) and adult dogs of small decorative breeds (lapdogs, dachshunds, Spitz, terriers, etc.) - 1 cm3;
- cats - 1 cm3.
Rabikan anti-rabies vaccine for cats and dogs
Rabies is a fatal disease caused by a specific pathogen, the rhabdovirus Rabies lyssavirus.
This is a typical zoonotic infection. The manifestation of its clinical signs means that there is no chance of salvation. Separately, it is worth considering a remedy that can help - Rabican, but this is a little lower. The main source of rhabdovirus is zoophages (carnivores). Therefore, vaccination of dogs and other domestic animals is mandatory and is carried out free of charge in state veterinary institutions.
Don't miss the chance to reliably protect yourself and your loved ones from this merciless disease! After all, a person usually becomes infected with the rabies virus after being bitten by a sick four-legged family pet.
Remember: medicine knows of only a few cases of recovery of people who were given this terrible diagnosis. So all cat and dog owners should unconditionally vaccinate their pets.
But which vaccine should you choose?
Rabies vaccination for kittens, cats and cats
Kittens begin to be vaccinated against rabies, just like puppies, at three months of age.
Revaccination after 21 days is not required. In the future, vaccination is done annually. The choice of vaccines for cats is slightly smaller than for dogs.
The most popular is the same Dutch drug Nobivac Rabies, which we wrote about above. It is perfectly combined with complex vaccines for cats from the same company: Nobivac Tricat against rhinotracheitis, calcivirosis and panleukopenia and Nobivac Forcat - rhinotracheitis, calcivirosis, panleukopenia and chlamydia.
You can also use the French complex vaccine Quadricat, which provides protection not only against rabies, but also calcivirosis, rhinotracheitis and panleukemia.
Your veterinarian will help you choose a drug and create an individual vaccination schedule.
Biological properties
- Being an effective means of preventing rabies in dogs, it forms super-active immunity to the rhabdovirus Rabies lyssavirus.
- After a single injection, immune resistance is developed already on days 5-7, which reaches its final formation after 30-40 days and persists for 12 months.
- A two-time injection with an interval of 30-50 days will ensure immunity stability at the level of 0.5 IU for at least 2 years.
- The drug is completely harmless: not a single case of any side effects has been recorded.
Application procedure
It is prohibited to use the dog rabies vaccine if infection with rhabdovirus is suspected.
The simultaneous use of Rabican in combination with other immunoprophylactic drugs, as well as its administration before/after 10 days during vaccination with antiviral drugs, is prohibited.
Vaccination of kittens and puppies is carried out starting from two months of age. Subcutaneous injection is performed in the withers area in compliance with antiseptic requirements:
- the injection site must be wiped with 70% ethyl alcohol or another antiseptic;
- A separate needle should be used for each pet.
The dosage of the drug depends on the dimensions:
- one-year-old puppies and miniature dogs are given 1 ml of vaccine;
- large dogs - 2 ml;
- cats (with any parameters) - 1 ml.
Before use, the contents of the bottle are diluted with distillate, restoring the dry vaccine to the volume indicated on the label.
Preventive measures against rabies in dogs consist of eradicating the disease within settlements and improving the health of areas infected with rabies infection.
In areas free from rhabdovirus, a single preventive immunization is carried out. Re-introduction of "Rabican" is carried out after a year; further revaccination scheme is 1 time every 2 years. If rabies infection has been introduced into these areas, then the animals are immunized without observing the one-year interval between the 1st and 2nd vaccinations.
In unfavorable areas, immunization is carried out twice with a break of 30-50 days. After this, a single revaccination is carried out once every 2 years. Those pets that have been vaccinated previously are vaccinated once every 2 years.
Emergency (forced) therapeutic and prophylactic vaccination in case of suspected infection is carried out within 48 hours. "Rabican" is administered twice with a two-week break in the dosages recommended for immunization.
Recommendations
Persons who take part in vaccination wear special clothing. The use of a robe, rubber boots, and gloves is implied. They wear closed glasses.
If persons have respiratory diseases, disorders of the functional functioning of the gastrointestinal tract, or allergic manifestations, they are not allowed to undergo the procedure.
If the vaccine is accidentally administered to a person, immediately treat the affected area with alcohol and immediately seek qualified medical help.
Note! If you see that there is no marking on the bottle or the integrity is broken, the color shade has changed, then you should not use the medicine. After immediate opening, no more than 4 hours pass.
Release form, composition and packaging
International nonproprietary or chemical name:
Anti-rabies inactivated dry culture vaccine from the Shchelkovo-51 strain for dogs and cats (Rabican)
Marketing authorization holder:
FKP "Shchelkovsky Biocombinat", 141142, Moscow region, Shchelkovo district, Biokombinat village
Developer:
FKP "Shchelkovsky Biocombinat", 141142, Moscow region, Shchelkovo district, Biokombinat village
Manufacturer:
FKP "Shchelkovsky Biocombinat", 141142, Moscow region, Shchelkovo district, Biokombinat village
Dosage form:
lyophilisate for the preparation of injection suspension
Qualitative composition and quantitative composition of active ingredients and qualitative composition of excipients:
The vaccine is made from a fixed rabies virus strain “Shchelkovo-51” inactivated by p-propiolactone with the addition of 33.3% sucrose-neptone-gelatin stabilizer.
Dosage:
2 cm3 (1 or 2 doses), 4 cm3 (2 or 4 doses), 10 cm3 (5 or 10 doses)
Quantity in consumer packaging:
2 each; 4 or 10 cm3 (1; 2; 5 doses for large breed dogs or 2; 4; 10 doses for cats, puppies and adult dogs of small decorative breeds, respectively) in bottles
Anti-rabies inactivated dry culture vaccine from the Shchelkovo-51 strain, 1 dose. (SumBF)
- 0
- ATC-vet classification code:
QI07AA – Inactivated viral vaccines. Dogs
- Types of animals:
cows, bulls, heifers, calves, horses, foals, pigs, piglets, sheep, lambs, goats, kids, dogs, puppies, cats, kittens, rabbits, fur-bearing animals
- Registration certificate (Ukraine):
ВВ-00145-02-09
- Description
- Characteristics
Composition: inactivated cultural vaccine strain “Shchelkovo-51” of a fixed rabies virus, which is propagated in a continuous cell culture VNK-21, inactivated by B-propiolactone (phenol or formaldehyde), adsorbed by aluminum hydroxide gel. Pharmaceutical form: dry porous mass of yellowish-brown color, which easily dissolves in sterile distilled water. Immunobiological properties: immunity in animals is formed on the 10th day and lasts for two years. Type of animals: dogs, cats, sheep, goats, cattle, pigs, horses. Indications: For preventive and forced vaccinations of animals against rabies in safe, endangered and disadvantaged farms. For preventive immunization of animals that are vaccinated against rabies for the first time, the vaccine is administered twice with an interval of 20-30 days. Animals previously vaccinated against rabies are given the vaccine once. Forced vaccination is carried out no later than 48 hours after possible infection of animals. The vaccine is administered three times in the above doses with an interval of 7 days. Horses, pigs, large and small cattle, cats are vaccinated from the age of three months, and dogs from the age of two months. Revaccination is carried out once every two years. Contraindication: Animals that are sick or suspected of having rabies are prohibited from being vaccinated. In order to identify contraindications, the doctor (paramedic) examines the animals on the day of vaccination. Caution when using: it is prohibited to use the drug after the expiration date or if the storage temperature is not observed. Vials (ampoules) with a vaccine containing foreign impurities, with cracks, with damaged packaging, without labels, not used on the day it was opened - are rejected and neutralized by boiling for 30 minutes. Interaction with other agents: 10 days before vaccination and for 10 days after using the vaccine, it is not recommended to treat animals with antibiotics, as well as against infectious, invasive diseases and skin parasites. Special instructions: Vaccination is carried out in compliance with aseptic rules. The injection site is wiped with 70% alcohol. Syringes and needles are sterilized by boiling for 30 minutes before and after use. Method of administration and doses: The vaccine is administered subcutaneously to large and small cattle, horses, dogs, cats, and intramuscularly to pigs. The vaccine is administered in doses per injection: large dogs (shepherds, St. Bernards, etc.) - 3 cm³; puppies 2 months of age and adult dogs of small decorative breeds (lapdogs, dachshunds, Spitz, terriers, etc.) - 1 cm³; cats - 1 cm³; sheep and goats - 3 cm³; cattle - 5 cm³; horses - 5 cm³; pigs - 2 cm³. Side effects: in vaccinated animals, a decrease in appetite is allowed for 1-2 days after vaccination. Withdrawal period: 14 days. Shelf life: 12 months. Storage conditions: in a dry, dark place at a temperature not exceeding 10 °C. Freezing is not allowed. Keep out of the reach of children. Packaging: sterile vials with a capacity of 10 cm3 from 1 cm3 to 8 cm3 of vaccine. Additional information: If the drug does not meet the requirements of the package insert or complications arise, the use of this series is immediately stopped and the State Scientific Control Institute of Biotechnology and Strains of Microorganisms (GNKIBSHM) and the supplier (manufacturer) are informed. Simultaneously with the messenger, three unopened bottles of this series of the drug are sent to the State Scientific Research Institute of Biomedical Medicine according to the “Instructions on the procedure for filing complaints for biological drugs that are intended for use in veterinary medicine” dated 06/03/98 No. 2 to the address: 03151, Kiev, Donetskaya St., 30 GNKIBSHM. Name and address of the manufacturer: State Enterprise “Sumy Biological Factory”, 40021, Ukraine, Sumy, st. Gamaleya, 25.
| ATC-vet classification code | QI07AA – Inactivated viral vaccines. Dogs |
| Types of animals | cows, bulls, heifers, calves, horses, foals, pigs, piglets, sheep, lambs, goats, kids, dogs, puppies, cats, kittens, rabbits, fur-bearing animals |
| Registration certificate (Ukraine) | ВВ-00145-02-09 |
| Active ingredients | inactivated rabies virus |
Tags: inactivated rabies virus