Mechanism of action of Cefazolin
The drug blocks the activity of cell membrane proteins of pathogenic gram-positive and gram-negative bacteria, preventing their reproduction in tissues and biological fluids of the body. Cefazolin is effective against various types of pathogens, including:
- streptococci;
- Klebsiella;
- protea;
- Treponema;
- enterobacteria;
- gonococcus;
- Haemophilus influenzae.
After injection, the active substance interacts with plasma proteins, penetrates bone tissue, lymph, synovial, pleural and other fluids. The maximum concentration in the body is observed after 2–2.5 hours. Cefazolin is not metabolized, is filtered by the kidneys and excreted in the urine. After intravenous injections, the elimination period is about 3–4 hours. After intramuscular administration, it remains in the body for up to 24 hours. Impaired kidney function slows down filtration and elimination of the antibiotic.
Cefazolin: indications for use
This semi-synthetic antibiotic has an effect on most unicellular microorganisms, gram-positive and gram-negative bacteria. It remains in the blood plasma for 12 hours, reaching its highest concentration 1 hour after administration intramuscularly or intravenously. Used in case:
- Staphylococcus, syphilis, gonorrhea, sepsis
- Inflammation of the abdominal cavity
- Infections of the biliary and urinary tract, musculoskeletal system, ENT organs, respiratory system
- Blood poisoning
- To prevent complications after surgery
Side effects are most often observed in people who have individual intolerance to cephalosporins and penicillins, but the body may react with increased sensitivity in the nervous system (convulsions), digestive, urinary and skin manifestations (itching, inflammation, swelling). With prolonged use, dysbacteriosis develops.
- Due to the fact that the drug is excreted by the kidneys, for any pathologies of this organ, the use of Cefazolin should be agreed with the attending physician, and the dosage is always reduced, the drug is administered once to prevent its accumulation in the kidneys.
- The drug cannot be used during pregnancy and lactation, as well as in children under 1 year of age, and in premature infants. The use of Cefazolin for intestinal diseases is undesirable.
For what pathologies is Cefazolin indicated?
Antibiotic injections are prescribed:
- for infectious lesions of the urinary system, liver, gall bladder;
- infectious bronchitis and pneumonia;
- for inflammatory diseases of the abdominal cavity and pelvic organs;
- with peritonitis and sepsis;
- for infectious complications after surgery;
- with mastitis;
- skin infections;
- sexually transmitted diseases.
The drug can be used from the age of 1 month.
How to prepare Cefazolin for injection: instructions
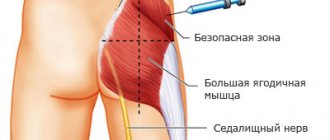
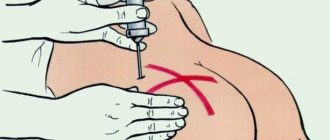
Since the main form of release of this antibiotic is powder, it requires preliminary preparation. Sterile water for injection is mainly used, but sometimes it is diluted with novocaine or lidocaine, thereby reducing the sensitivity of the injection area. When administered intramuscularly, the injection of Cefazolin is always painful.
- When Cefazolin is administered intravenously through a dropper, 1 g of powder is dissolved in isotonic sodium chloride or glucose solution with a 5% concentration. Liquid volume - 250 ml. The duration of the procedure is 30 minutes; it is performed twice for an adult. Maximum dosage - 6 g.
- When Cefazolin is administered intramuscularly, through an injection, 0.5-2 g of powder accounts for 3 ml of water for injection. Sodium chloride can also be used. For an intravenous injection, the volume is increased to 10 ml.
- How to dilute Cefazolin with novocaine? For this purpose, a 1% solution of novocaine (or a lower concentration) is used; for 1 g of Cefazolin there is 5 ml of novocaine.
The system for diluting powder with liquid looks like this:
- The foil is removed and the lid is treated with medical alcohol.
- The liquid is poured into a bottle with powder.
- The bottle is shaken to dissolve the granules.
- The resulting solution is injected into a syringe.
Side effects of the drug
Cefazolin injections cause a local painful reaction: a strong burning sensation at the injection sites that lasts for several minutes. This effect does not require discontinuation of the drug. Other side effects:
- dyspepsia: nausea, stomach cramps, loss of appetite;
- stool disorder;
- fungal infections of the skin and mucous membranes;
- changes in blood count: thrombocytopenia, leukopenia, increased liver transaminases;
- aching pain in the joints.
If you are allergic to the drug, in rare cases, Quincke's edema develops, skin itching, fever, and urticaria are possible. In case of overdose, cardiotoxic shock, arrhythmia, and vomiting develop.
Is it possible to take Cefazolin with alcohol?
The instructions for the drug do not contain information about how Cefazolin and alcohol interact, which for many (and Cefazolin is a fairly popular antibiotic) confirms the possibility of drinking alcohol during treatment with Cefazolin.
Well, it is everyone’s personal right to put their body to the test, accidentally or intentionally provoking potentially dangerous situations. However, doctors still advise avoiding such a combination: no one ever knows whether Cefazolin and alcohol will give consequences after being taken together or not.
What are the risks and potentially dangerous situations associated with taking Cefazolin and drinking alcohol?
According to foreign studies, when combining an antibiotic with alcohol, due to the presence of the N-methylthiodiazole (NMTD or 1-MTD) side chain, Cefazolin poses a risk of developing Antabuse-like reactions (similar to those in treatment, for example, Torpedo or Esperal). This is due to the inhibition of NMTD aldehyde dehydrogenase, an enzyme necessary for the breakdown of ethanol in the liver. This fact helps to understand whether Cefazolin can be taken with alcohol.
According to the annotation, Cefazolin does not undergo biotransformation in the body, is excreted through the kidneys, and therefore there is no risk that it will directly “meet” alcohol in the liver. But as always there are a few “buts”.
First. Cefazolin belongs to the group of cephalosporins that are excreted through the kidneys (this group also includes drugs that are excreted through the liver and through the liver and kidneys). The rate of its elimination (and therefore the effectiveness of action) is influenced by the indicators of tubular secretion. This is a special mechanism of the transport function of the kidneys, which allows xenobiotics, including antibiotics, to be quickly eliminated from the body. Alcohol has a toxic effect on the kidneys, including disrupting the processes of renal excretion. This leads to a violation of pharmacokinetics (how the drug behaves in the body), and through it to a violation of the pharmacodynamic properties (that is, the effects the drug has on the body).
The second “but” is a very broad topic concerning possible reactions between Cefazolin and alcohol as factors in the development of tubulointerstitial nephritis. Both alcohol and antibiotics often cause this kidney pathology, which leads to functional and morphological changes in the kidneys.
Before administration, cefazolin is diluted with a solvent - usually a solution of procaine (novocaine) in 0.25 - 0.5% concentration or saline solution. Alcohol increases the acidity of the body, including urine, which in turn increases the rate of excretion of novocaine. It is eliminated from the body faster, which can affect absorption and, subsequently, the effectiveness of the antibiotic.
Recently, there has been an increase in the resistance of microorganisms to cephalosporin antibiotics, including Cefazolin. This is due to the acquisition by microorganisms of some properties typical of penicillin-binding protein. Thus, at present, cephalosporins are ineffective or negligibly effective against some Citrobacter, Enterobacter, Escherichia, Proteus, Pseudomonas aeruginosa and Serratia.
Use of Cefazolin: instructions
Injections of the drug are prescribed in short courses: no more than 7–10 days with daily administration. The exact regimen is selected by the doctor depending on the type of disease and its severity. Daily dosage:
- for adults: from 1 to 6 years;
- for children: 25–30 mcg of powder per 1 kg of body weight, for critical conditions: 100 mcg per 1 kg of weight.
For patients with impaired renal and liver function, the dosage is halved.
Injections are administered 2–3 times a day. Intravenous: through a syringe for 5 minutes or infusion through a dropper.
Rules for diluting the solution:
- for intramuscular injections: the powder is diluted with distilled water, saline;
- for intravenous: sodium chloride solution;
- for injection droppers: glucose solution.
The powder and liquid are combined in one container and shaken thoroughly until completely dissolved. Mixing Cefazolin with other antibiotics is prohibited. To reduce the pain of injections, the drug can be diluted with anesthetics: Novocaine, Lidocaine, using them instead of water or saline. The drug prepared for injection cannot be stored; it must be used immediately.
How to dilute Cefazolin for children?
The dosage of this antibiotic for children should be prescribed by a doctor, but in a critical situation you can calculate it yourself and also give your baby an injection yourself.
- For each kg of net weight, take from 25 to 50 mg of Cefazolin, depending on the sensitivity of the body.
- A bottle of powder for children should contain 0.5 g of antibiotic for the most accurate dosage. At higher concentrations (1 g, 2 g), 10 and 20 ml of liquid are taken, respectively.
- Water for injection or novocaine is also added to the bottle, and the required number of ml for administration is removed with a syringe.
The above instructions and recommendations for the use of Cefazolin do not replace consultation with a specialist. If any side effects occur, use of the antibiotic should be discontinued. Remember that Cefazolin solution can only be administered if it is completely clear.
Use of the drug Cefazolin
Cefazolin is administered intramuscularly and intravenously (stream or drip). For intramuscular administration, a solution of the drug is prepared ex tempore , the contents of the bottle are dissolved in 4–5 ml of isotonic sodium chloride solution or sterile water for injection and injected deep into the muscle. For intravenous jet administration, a single dose of the drug is dissolved in 10 ml of isotonic sodium chloride solution and administered slowly over 3–5 minutes. When administered intravenously, the drug (0.5–1.0 g) is dissolved in 100–250 ml of isotonic sodium chloride solution or 5% glucose solution; the injection is carried out over 20-30 minutes (injection rate - 60-80 drops per 1 minute). The daily dose of the drug for adults is 1–4 g (sometimes more) and depends on the severity of the infection, the type of pathogen and its sensitivity to the antibiotic. A single dose of the drug for adults for infections caused by gram-positive microorganisms is 0.25–0.5 g every 8 hours. For moderate respiratory tract infections caused by pneumococci and urinary tract infections, the drug is prescribed at 0.5–1.0 g every 12 hours. For diseases caused by gram-negative microorganisms, the drug is prescribed 0.5–1.0 g every 6–8 hours. For severe infections (sepsis, endocarditis, peritonitis, destructive pneumonia, acute hematogenous osteomyelitis, complicated urological infections) daily the dose of the drug can be increased to 6 g (maximum) with an interval between administrations of 6–8 hours. For children over 1 month of age, the drug is prescribed in a daily dose of 20–50 mg/kg body weight (in 3–4 doses), for severe infections — 100 mg/kg (maximum dose). If renal excretory function is impaired in adults, the treatment regimen is adjusted by reducing the dose of the drug and increasing the intervals between doses. The initial dose of the drug, regardless of the degree of renal impairment, is 0.5 g. Recommended doses for the treatment of adults with impaired renal function: - with blood urea nitrogen 20-34 mg% and creatinine clearance 70-40 ml/min, dose of the drug for mild infection or moderate infection is 0.25-0.5 g every 12 hours, for severe infection - 0.5-1.25 g every 12 hours (half-life - 3-5 hours); – with blood urea nitrogen 35–49 mg% and creatinine clearance 40–20 ml/min, the dose of the drug for mild or moderate infection is 0.125–0.25 g every 12 hours, for severe infection — 0.25–0.6 d every 12 hours (half-life - 6-12 hours); – with blood urea nitrogen 50-75 mg% and creatinine clearance 20-5 ml/min, the dose of the drug for mild or moderate infection is 75-150 mg every 24 hours, for severe infection - 150-400 mg every 24 hours (period half-life – 15–30 hours); – with blood urea nitrogen 75 mg% and creatinine clearance 5 ml/min, the dose of the drug for mild or moderate infection is 37.5-75 mg every 24 hours, for severe infection - 75-200 mg every 24 hours (half-life - 30–40 hours). If renal function is impaired in children, the usual single dose of Cefazolin is first administered, then subsequent doses of the drug are adjusted taking into account the degree of renal failure. If renal function is impaired in children, the usual single dose of Cefazolin is first administered, then subsequent doses of the drug are adjusted taking into account the degree of renal failure. In children with moderately severe renal impairment (creatinine clearance from 70 to 40 ml/min), the daily dose is 60% of the daily dose of the drug, which is used for normal renal function, and is divided into 2 administrations; with creatinine clearance from 40 to 20 ml/min, the dose of the drug is 25% of the norm and is divided into 2 injections; in case of significant impairment of renal function (creatinine clearance from 20 to 5 ml/min), the daily dose is 10% of the norm with an interval between doses of 24 hours. Cefazolin solutions should not be mixed with other antibiotics in the same syringe or in the same solution for intravenous infusion. The duration of treatment depends on the form and severity of the disease and is ≥7–10 days.
Pharmacological properties of the drug Cefazolin
Pharmacodynamics. Cefazolin has a wide spectrum of antimicrobial (bactericidal) action. Active against gram-positive microorganisms ( Staphylococcus spp ., producing and not producing penicillinase, most strains, including pneumococci, Corinebacterium diphtheriae ); gram-negative microorganisms ( Escherichia coli, Salmonella spp., Shigella spp., Proteus mirabilis, Klebsiella spp., Haemophylus influenzae, Enterobacter aerogenes, Neisseria gonorrhoeae ). Indole-positive strains of Proteus ( P. morgani, P. vulgaris, P. rettgeri ), Pseudomonas aeruginosa are resistant to the antibiotic; does not affect rickettsia, viruses, fungi, protozoa. Like penicillins, it inhibits the synthesis of bacterial cell walls. Pharmacokinetics. When administered intramuscularly, the drug is rapidly absorbed, reaches a maximum concentration in the blood after 1 hour and is retained in an effective concentration in the blood plasma for 8–12 hours. It is excreted mainly (approximately 90%) by the kidneys unchanged. Penetrates through the placental barrier into amniotic fluid and umbilical cord blood. Found in very low concentrations in breast milk. The drug penetrates well through the inflamed synovium into the joint cavity. With intravenous administration, a higher concentration is formed in the blood, but the drug is released faster (half-life is about 2 hours).
Special instructions for the use of Cefazolin
In patients with reduced renal function, it is necessary to select the dose and intervals between administrations of cefazolin depending on the degree of functional failure. If renal function is impaired, constant monitoring of the level of cefazolin in the blood serum will ensure the safety of the drug. If signs of allergic reactions appear, administration of the drug should be discontinued; it is necessary to prescribe appropriate symptomatic treatment. It is possible to develop cross-allergic reactions with other cephalosporin antibiotics, as well as in some cases with penicillin antibiotics. Studies have not revealed any adverse effects of cefazolin on the fetus. Cefazolin does not affect the ability to drive vehicles or operate potentially dangerous machinery.