Cost of vaccination
| Service | Price | Note |
| RotaTech, USA | 3400 | from 6 to 32 weeks |
Almost every second mother who has one child, and every first mother who has more than one child, knows what “intestinal flu” or rotavirus is.
If the terminology is unfamiliar, then, in any case, absolutely everyone has gone through this disease! In a simplified way, this disease can be characterized as follows: the onset is sudden, the temperature reaches 39 and above, repeated bouts of vomiting, then diarrhea occurs, which lasts on average about 5 days. Naturally, the severity of the disease depends on the general condition of the body and its immunity. The first time the disease is most often quite severe, later, as a rule, it is easier.
Although the entrance gate for rotavirus infection is the oral cavity, from where viruses enter the underlying parts of the intestinal tract, “rotavirus” received its name for its resemblance to a wheel (translated from Latin rota - “wheel”).
Rotaviruses infect almost every child (!) before they reach the age of 3 - 5 years and are the world's main cause of severe diarrhea with dehydration of the body in children under 5 years of age. In developed countries with high incomes, the first episode of infection usually occurs between 2 and 5 years of age, although most cases occur in infants (65% of cases occur before age 1 year).
Every year in the period before the introduction of the vaccine (1986-2000), more than 2 million children worldwide were hospitalized for rotavirus infection. The widespread spread of rotavirus infection even in conditions of high hygienic standards indicates a high level of transmission of this virus.
Currently, there is no specific therapy for rotavirus infection. As with other childhood diarrheas, the main treatment is to restore fluid loss to prevent dehydration.
Vaccinations against rotavirus infection are currently carried out only in the first year of life.
This causes some difficulties.
Practical medical experience in vaccine prevention shows that parents of their first child often do not vaccinate their baby, because are not fully informed about the existence of rotavirus infection and vaccination against it, and are not sure of this need. The desire to vaccinate with Rotatek usually appears when the child is already 2-3 years old or more. Unfortunately, vaccination against rotavirus infection is not provided at this age.
And most often, in children's medical clinics, vaccination against rotavirus infection is carried out for children who already have older brothers and sisters. In other words, parents have already encountered this problem and want to protect their “new” baby from rotavirus infection. Therefore, the most complete and reliable information about the possibility of vaccination against rotavirus infection is the key to preventing this disease in children. In addition, the vaccination is painless for the baby (drops in the mouth), and can be combined with vaccinations with other vaccines.
In European countries, as well as in the United States, vaccination against rotavirus infection has been introduced into the national calendar and has been provided to children since the 1990s.
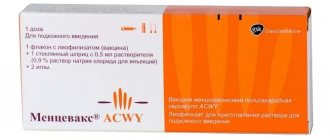
, USA (RotaTeq®, Merck& Co., USA).
Rotavirus vaccine
The RotaTek rotavirus vaccine has been used all over the world since 2006, and in Russia since 2012. It has passed all the necessary clinical trials and is recommended for use by the Ministry of Health of the Russian Federation. Refers to polyvalent vaccines and includes 5 serotypes of the virus. The composition includes individual sections of RNA viruses that cause the necessary immunological response. The right combination allows you to protect the body from the most common serotypes. Numerous clinical trials have proven that vaccination against rotavirus with the RotaTek vaccine promotes the formation of specific immunity not only to the contained serotypes, but also to other varieties of the virus.
Vaccination scheme
Three doses of the vaccine are administered. The first dose of the RotaTek vaccine should be administered as soon as possible after the child reaches the age of 1.5 months (6 weeks). Subsequent doses are given 4 to 10 weeks apart, with the final dose of vaccine given before the child reaches 32 weeks (8 months) of age.
Standard recommended RotaTek vaccination schedule: 2-3-4.5 months.
The RotaTek vaccine can be used in premature babies born at at least 25 weeks of gestation. The vaccine should be administered to such children no earlier than 6 weeks after birth.
RotaTec®
In temperate climates, rotavirus gastroenteritis is a seasonal disease with an increase in incidence (epidemics) in the winter months. If left untreated, rotavirus gastroenteritis can lead to fatal dehydration.
Efficiency
According to clinical studies, the effectiveness of the RotaTek® vaccine has been demonstrated against gastroenteritis caused by rotaviruses of genotypes G1 P[8], G2P[4], G3P[8], G4P[8] and G9P[8].
The effectiveness of the RotaTek® vaccine was studied in 2 parameters in a placebo-controlled Efficacy and Safety Study (REST):
1. In 5673 vaccinated children (2834 children in the vaccine group), effectiveness was measured as a reduction in the number of cases of rotavirus gastroenteritis caused by the G genotypes included in the vaccine (G1-G4). which developed 14 days after receiving the third dose of the vaccine and throughout the first full rotavirus season after vaccination.
2. In 68,038 vaccinated children (34,035 children in the vaccine group), effectiveness of protection was measured as a decrease in the number of hospitalizations and emergency room visits for rotavirus gastroenteritis, starting 14 days after receiving the third dose of vaccine.
The results of these studies are presented in tables.
| Reduction in the number of cases of rotavirus gastroenteritis over one full season after vaccination (RotaTek® vaccine group n=2834) (% [95% confidence interval (CI)]) | ||||||
| Efficacy against rotavirus gastroenteritis of any severity depending on the genotype | ||||||
| Severe* disease (G1-G4) | Any degree of severity (G1-G4) | G1 | G2 | G3 | G4 | G9 |
| 98,0% | 74,0% | 74,9% | 63,4% | 82,7% | 48,1% | 65,4% |
| [88,3; 100,0]† | [66,8; 79,9]† | [67,3; 80,9]† | [2,6; 88,2]† | [<0; 99,6] | [<0; 91,6] | [<0; 99,3] |
* Severe was defined as a score greater than 6 out of 24 on a validated clinical scoring system based on the intensity and duration of symptoms (fever, vomiting, diarrhea, behavioral changes).
†Statistically significant.
| Reduction in the number of hospitalizations and emergency room visits for rotavirus gastroenteritis over 2 years after vaccination (RotaTek® vaccine group n=34035) (% [95% CI]) | |||||
| G1-G4 | G1 | G2 | G3 | G4 | G9 |
| 94,5% | 95,1% | 87,6% | 93.4% | 89,1% | 100% |
| [91,2; 96.6] † | [91,6; 97,1]† | [<0; 98,5] | [49,4; 99,1]† | [52,0; 97,5]† | [69.6; 100,0]† |
†Statistically significant.
The reduction in the number of cases of rotavirus gastroenteritis caused by genotypes G1-G4 during the second rotavirus season after vaccination was 88.0% [95% CI: 49.4; 98.7] for severe disease and 62.6% [95% CI: 44.3; 75.4] for a disease of any severity.
Efficacy against rotavirus genotypes G2P[4], G3P[8], G4P[8] and G9P[8] was calculated for fewer cases than for G1. Efficacy against G2P[4] genotypes. most likely due to the presence of the G2 reassortant in the vaccine.
In a combined post hoc analysis of the REST study and another phase 3 study, vaccine efficacy against rotavirus gastroenteritis (any grade) caused by serotypes G1, G2, G3, and C4 was 61.5% [95% CI: 14.2; 84.2] among children who received the third dose of the drug at the age of 26 to 32 (inclusive) weeks.
An additional study was conducted in Finland as part of the REST study. The Finnish Extension Study (FES) followed a group of 20,736 children who had previously participated in the REST study. In the FES study, children were followed for up to 3 years after vaccination.
The REST study reported 403 cases of rotavirus gastroenteritis associated with genotypes G1-G4 and G9 (20 cases in the vaccine group and 383 in the placebo group). In the FES study, an additional 136 cases were observed (9 in the vaccine group and 127 in the placebo group). The number of cases for each group in the FES study was 31% and 25% of the sum of cases for the corresponding groups in the two studies.
Based on pooled REST and FES data, the reduction in hospitalizations and emergency department visits for rotavirus gastroenteritis within 3 years of vaccination was 94.4% (95% CI: 91.6, 96.2) for genotypes G1-G4. 95.5% (95% CI: 92.8; 97.2) for the GI genotype, 81.9% (95% CI: 16.1; 98.0) for the G2 genotype, 89.0% (95% CI : 53.3; 98.7) for genotype G3, 83.4% (95% CI: 51.2; 95.8) for genotype G4 and 94.2% (95% CI: 62.2; 99.9 ) for genotype G9. During the third year, there were no visits to medical care for rotavirus gastroenteritis in the vaccine group (3112 children), and 1 (untypeable) case was noted in the placebo group (3126 children).
Clinical studies have confirmed that the full 3-dose course of vaccination should be administered to achieve the required level and duration of protection against rotavirus gastroenteritis (see CFIOCOB APPLICATIONS AND DOSES). However, a retrospective analysis of the data showed that even before completion of the full course of vaccination, the number of cases of rotavirus gastroenteritis with a severity that would require hospitalization or emergency care was reduced (approximately 14 days after the first dose).
Efficacy in premature infants
In the REST study, RotaTec® vaccine was administered to approximately 1,000 infants born between 25 and 36 weeks' gestation. The effectiveness of the RotaTek® vaccine in this subgroup did not differ from the subgroup of children born at term.
Post-marketing observational safety study
In a large prospective post-marketing observational study conducted in the United States. The risk of Kawasaki disease was analyzed in 85,150 children who received one or more doses of RotaTek® vaccine (17,433 patient-years of observation).
There was no statistically significant difference in the incidence of Kawasaki disease between 0 and 30 days after vaccine administration compared with the incidence in the general population. In addition, there was no statistically significant increase in the risk of this side effect when observed within 30 days after administration of the RotaTek® vaccine compared to the control group of children who received only the DTP vaccine (62,617 children, 12,399 patient-years of observation). One confirmed case was reported in children receiving RotaTek® vaccine compared to one case in children receiving DTP (relative risk 0.7; 95% CI: 0.01, 55.56). Therefore, no additional safety risk was identified relative to the overall safety profile.
Efficacy Research Data
Post-registration studies confirming the effectiveness of preventing rotavirus gastroenteritis.
| Study design (country) | Study population | Endpoints | Efficacy in % [95% CI] | Rotavirus incidence seasons |
| Case Database (USA) | 33140 vaccinated 26167 unvaccinated Age ≥7 months 3 doses received | Hospitalizations and emergency visits for rotavirus gastroenteritis | 100% [87; 100] | 2007-2008 |
| Outpatient visits for rotavirus gastroenteritis | 96% [76; 100] | |||
| Hospitalizations and emergency visits for all types of gastroenteritis | 59% [47;68] | |||
| Cohort study (France) | 1895 vaccinated with 3 doses 2102 unvaccinated Age <2 years | Hospitalizations for rotavirus gastroenteritis | 98% [83; 100] | 2007-2008 2008-2009 |
| Case-control study (USA) | 402 cases 2559 controls* Age <8 years 3 doses received | Hospitalizations and emergency visits for rotavirus gastroenteritis | 80% [74:84] | 2011-2012 2012-2013 |
| Strain specific | ||||
| — G1 P[8] | 89% 155:97] | |||
| - G2P[4] | 87% [65;95] | |||
| - G3P[8] | 80% [64:89] | |||
| — G12P[8] | 78% [71:84] | |||
| Age specific | ||||
| — 1st year of life | 91% [78:96] | |||
| — 2nd year of life | 82% [69:89] | |||
| — 3rd year of life | 88% [78:93] | |||
| — 4th year of life | 76% [51:88] | |||
| — 5th year of life | 60% [16:81] | |||
| — 6th-7th years of life | 69% [43:84] |
* Control - rotavirus-negative cases of acute gastroenteritis.
Immunogenicity
The mechanism of immune protection of the body by the RotaTek® vaccine against rotavirus gastroenteritis has not been fully studied. The relationship between the level of antibodies to rotaviruses after vaccination with rotavirus vaccines and the degree of protection against rotavirus gastroenteritis has not been established. According to data from phase 3 clinical studies, after a full course of vaccination, consisting of three doses of the RotaTek® drug, a significant increase in the level of anti-rotavirus IgA was observed in the serum of 92.5-100% of vaccinated people. The vaccine causes an immune response, i.e. formation of serum neutralizing antibodies to five human rotavirus proteins expressed on vaccine reassorts (Gl. G2, G3, G4 and P1 A [8]).