Brucellosis is one of the infectious diseases characteristic of animals, but capable of infecting the human body. This pathological condition affects vital organ systems, in particular, the structural elements of the reproductive, cardiovascular, nervous system and musculoskeletal system.
The causative agent of brucellosis is a bacterium of the same name, which enters the human body through nutrition and contact from cattle, pigs, hares, deer, and the like. Today you can protect yourself from the disease through routine vaccination.
The modern vaccine against brucellosis is a reliable immune preparation that can protect a person from pathogenic microorganisms and prevent the development of symptoms of the disease, as well as its complications.
Composition and effect of brucellosis vaccine live dry
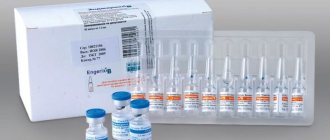
Live dry vaccine against brucellosis is a lyophilisate for preparing a suspension. The solution obtained during the preparation process is indicated for subcutaneous administration or scarification application to the epidermal integument.
The dry vaccine looks like a porous mass of white color with a yellowish tint. It is a lyophilized mixture of living microorganisms that are in a weakened state. To produce the drug, the vaccine strain Brucella abortus 19 BA is used.
Live brucellosis vaccine, dry
Each dose of the vaccine for subcutaneous use contains 0.5 ml of solution from 3.4 to 4.6 x 108 live pathogens. The suspension for scarification application is more concentrated. Every tenth of a milliliter contains from 4.0 x 109 to 1.6 x 1010 pathogenic microorganisms.
As a rule, one ampoule of the drug contains from four to ten doses of the vaccine for adult patients. After the vaccine is administered, the body develops reliable immune protection for up to a month, sufficient to prevent the pathological process.
Such anti-brucellosis immunity can persist in a person’s blood for up to 12 months, after which the patient is recommended to be revaccinated. The maximum amount of antibodies to brucellosis is determined in serum up to 5-6 months after the procedure.
Inactivated therapeutic brucellosis vaccine
Once cutaneously and subcutaneously.
One dose for cutaneous administration is 2 drops (contains 10 billion microbial cells), for subcutaneous administration - 0.5 ml (contains 400 million microbial cells). Revaccination is carried out after 10-12 months cutaneously, using half the dose (5 billion microbial cells).
Cutaneously: the drug is diluted with a 0.9% NaCl solution, which is added to the ampoule with a sterile syringe with a needle at the rate of 0.1 ml (2 drops) per 1 vaccination dose. The ampoule is shaken until a uniform suspension is formed. The dissolution time of the vaccine should not exceed 1 minute. The dissolved drug should not contain sediment or flakes. The outer surface of the middle third of the shoulder is treated with ethanol or a mixture of ethanol and ether; the use of disinfectant solutions is not permitted. After the ethanol and ether have evaporated, without touching the skin, apply 2 drops of the vaccine at a distance of 30-40 mm from each other, stretch the skin and use a sterile scarifier to make 6 cuts (3 longitudinal and 3 transverse) through each applied drop of vaccine, each 10 mm long with a distance between notches 3 mm. The notches should not bleed, blood should only come out. Using the flat side of the scarifier, rub the vaccine into the incisions for 30 seconds and allow to dry for 5 minutes.
When revaccinating, use half the dose, i.e. 1 drop of vaccine, through which 6 cuts are made.
S/c: the vaccination dose of the drug is 25 times less than with cutaneous vaccination; The vaccine is diluted at the rate of 12.5 ml of 0.9% NaCl solution per dose of the vaccine for cutaneous use (s.c. dose - 0.5 ml x 25 = 12.5 ml). The drug contained in the ampoule is dissolved in the same way as for cutaneous vaccination, after which the resulting suspension is transferred into a sterile injector bottle into which the required volume of solvent is added (for example, if the ampoule contains 7 cutaneous doses of the vaccine, then the contents should be suspended in 12.5 ml x 7, i.e. in 87.5 ml).
Vaccination is carried out in the area of the outer surface of the shoulder at the border between the upper and middle third. The injection site is treated in the same way as with the cutaneous route of administration. The vaccine is administered in a volume of 0.5 ml with the injector operating mode designed for subcutaneous administration (according to the Instructions for use of the BI-ZM injector and the PPI-2 anti-infective protector).
Before vaccination, the presence of specific immunity must be determined using one of the serological or skin-allergic reactions. Persons with a negative reaction are subject to vaccination.
When is a person vaccinated against brucellosis?
Vaccination against brucellosis is recommended for those people who quite often come into contact with animals, that is, their type of work is related to this.
First of all, the following categories of the population should be immunized against brucellosis bacteria:
- workers of farms and agricultural enterprises raising large and small livestock, pigs, etc.;
- people working at enterprises for the procurement, processing and storage of animal products obtained from farms where episodic outbreaks of brucellosis in animals have been recorded;
- slaughterers of livestock with brucellosis infection;
- veterinarians;
- hunters and foresters;
- children living in rural areas and farms, where there is always a risk of contact with an infected animal;
- animal science.
Laboratory workers who produce vaccines against brucellosis are also subject to vaccination, as they are always at increased risk of contracting the disease.
Farm owners today monitor the health of their livestock, and therefore take a responsible approach to animal vaccination, which includes vaccination against brucellosis. Naturally, for people working at such enterprises, the risk of contracting an infection is minimal, but it is still present.
bovine brucellosis vaccine
Class A61K39/10 Brucella; Bordetella, such as whooping cough
| combined vaccine including antigens of diphtheria, tetanus, acellular whooping cough, haemophilus influenzae and poliovirus, its use and production method - patent 2526214 (08/20/2014) | |
| a method for treating radiation, chemical and/or biological damage to the body and a method for producing globulins for the treatment of radiation, chemical and/or biological damage to the body - patent 2524612 (07/27/2014) | |
| method for emergency prevention and treatment of brucellosis - patent 2523392 (07/20/2014) | |
| method for differential epizootic assessment of brucellosis in cattle herds immunized with live vaccines from dissociated strains of Brucella - patent 2518308 (06/10/2014) | |
| method for producing acellular vaccine for immunoprophylaxis of whooping cough - patent 2504399 (01/20/2014) | |
| method for preventing brucellosis in animals - patent 2501567 (12/20/2013) | |
| polynucleotide sequence encoding the constructed pertactin protein, a vector including such a sequence, and vaccine compositions containing the pertactin protein or vector - patent 2499046 (11/20/2013) | |
| method of obtaining erythrocyte antibody diagnosticum for the indirect hemagglutination reaction (indirect hemagglutination reaction) for the purpose of indicating Brucella in pathological material - patent 2493871 (09.27.2013) | |
| method for preventing brucellosis in cattle - patent 2491091 (08/27/2013) | |
| method for obtaining brucellosis antigen for rose bengal test (rbp) - patent 2488119 (07/20/2013) |
Class C12N1/20 bacteria; nutrient media for them
| method for determining the sensitivity of pathogenic bacteria to complex antibacterial drugs - patent 2529711 (09/27/2014) | |
| biphasic transport nutrient medium for isolating and growing the brucellosis microbe - patent 2529364 (09/27/2014) | |
| nutrient medium for growing a consortium of nitrogen-fixing and phosphate-mobilizing microorganisms - patent 2528874 (09.20.2014) | |
| nutrient medium for growing a consortium of nitrogen-fixing and phosphate-mobilizing microorganisms - patent 2528873 (09/20/2014) | |
| strain of lactobacillus fermentum, which has a wide spectrum of antagonistic activity and a probiotic consortium of lactobacilli for the manufacture of bacterial preparations - patent 2528862 (09/20/2014) | |
| isolated strain (variants) that improves the health of ruminant animals, a method for its preparation, and a method for its administration to ruminant animals - patent 2528859 (09/20/2014) | |
| method for producing millerite using sulfate-reducing bacteria - patent 2528777 (09.20.2014) | |
| nutrient medium for growing a consortium of nitrogen-fixing and phosphate-mobilizing microorganisms - patent 2528744 (09/20/2014) | |
| nutrient medium for growing a consortium of nitrogen-fixing and phosphate-mobilizing microorganisms - patent 2528740 (09.20.2014) | |
| nutrient medium for the cultivation of legionella - patent 2528101 (10.09.2014) | |
Preparation for vaccination against brucellosis in humans
Before the vaccination procedure, a person does not need special preparation.
Directly on the day of the injection, the doctor conducts a survey of the patient and his general examination with the obligatory measurement of body temperature. If thermal indicators during examination are elevated, then vaccination should be postponed until they normalize.
Before vaccination, patients are recommended to undergo a general blood test to determine signs of hidden inflammation in the body.
This will help ensure that the condition of the vaccinated person is satisfactory and prevent the occurrence of post-vaccination complications. Doctors often decide on the need to conduct serological tests to determine the presence of specific immunity against brucellosis. The vaccine is given only to patients with a negative reaction.
Vaccination should be carried out four weeks before taking on a job that poses an increased risk of infection. This time is enough for the human immune system to form protective antibodies against the pathogen.
Brucellosis vaccine
Once cutaneously and subcutaneously.
One dose for cutaneous administration is 2 drops (contains 10 billion microbial cells), for subcutaneous administration - 0.5 ml (contains 400 million microbial cells). Revaccination is carried out after 10-12 months cutaneously, using half the dose (5 billion microbial cells).
Cutaneously: the drug is diluted with a 0.9% NaCl solution, which is added to the ampoule with a sterile syringe with a needle at the rate of 0.1 ml (2 drops) per 1 vaccination dose. The ampoule is shaken until a uniform suspension is formed. The dissolution time of the vaccine should not exceed 1 minute. The dissolved drug should not contain sediment or flakes. The outer surface of the middle third of the shoulder is treated with ethanol or a mixture of ethanol and ether; the use of disinfectant solutions is not permitted. After the ethanol and ether have evaporated, without touching the skin, apply 2 drops of the vaccine at a distance of 30-40 mm from each other, stretch the skin and use a sterile scarifier to make 6 cuts (3 longitudinal and 3 transverse) through each applied drop of vaccine, each 10 mm long with a distance between notches 3 mm. The notches should not bleed, blood should only come out. Using the flat side of the scarifier, rub the vaccine into the incisions for 30 seconds and allow to dry for 5 minutes.
When revaccinating, use half the dose, i.e. 1 drop of vaccine, through which 6 cuts are made.
S/c: the vaccination dose of the drug is 25 times less than with cutaneous vaccination; The vaccine is diluted at the rate of 12.5 ml of 0.9% NaCl solution per dose of the vaccine for cutaneous use (s.c. dose - 0.5 ml x 25 = 12.5 ml). The drug contained in the ampoule is dissolved in the same way as for cutaneous vaccination, after which the resulting suspension is transferred into a sterile injector bottle into which the required volume of solvent is added (for example, if the ampoule contains 7 cutaneous doses of the vaccine, then the contents should be suspended in 12.5 ml x 7, i.e. in 87.5 ml).
Vaccination is carried out in the area of the outer surface of the shoulder at the border between the upper and middle third. The injection site is treated in the same way as with the cutaneous route of administration. The vaccine is administered in a volume of 0.5 ml with the injector operating mode designed for subcutaneous administration (according to the Instructions for use of the BI-ZM injector and the PPI-2 anti-infective protector).
Before vaccination, the presence of specific immunity must be determined using one of the serological or skin-allergic reactions. Persons with a negative reaction are subject to vaccination.
Vaccine administration method and dosage
In humans and animals, the same dry vaccine against brucellosis is used during immunization. The only difference is the dosage of the drug used in different groups of patients.
There are two main ways to introduce a vaccine into the body:
- subcutaneous injection;
- cutaneous scarification injection.
When administering the vaccine subcutaneously, the drug must first be diluted with a 0.9% sodium chloride solution, which is added to the ampoule using a sterile syringe. After this, the ampoule should be shaken well until a homogeneous suspension is obtained. The resulting liquid is transferred into a sterile injector bottle with the required volume of solvent. The vaccination is done using an injector, following all points of the instructions for use of the drug.
The ideal place to inject the solution is the lateral surface of the upper third of the shoulder. Before the procedure, the injection site is treated with a disinfectant. For subcutaneous administration, the suspension is prepared in the same way as for the subcutaneous vaccination option. The graft is done on the outer surface of the middle third of the shoulder.
The site where the vaccine is applied is treated with an alcohol solution. After the alcohol product has evaporated, two drops of the drug are applied to the skin of the shoulder at a distance of 3-4 cm from each other. After this, the skin should be stretched and a sterile scarifier should be pulled through each drop six times, making notches in the form of a mesh, that is, three longitudinal and three transverse lines.
The cuts made should not bleed. Blood should appear only in droplets. Use the flat side of the scarifier to rub the solution into the incisions for 30 seconds. Then you need to let the liquid dry.
For revaccination, one drop of the product is applied to the skin of the shoulder instead of two.
Live brucellosis vaccine
Dosage regimen and method of administration
Vaccination is carried out once, cutaneously or subcutaneously.
One dose for cutaneous administration is 2 drops (0.1 ml) and contains 1x1010 m.k., for subcutaneous administration it is 0.5 ml and contains 4x108 m.k. Revaccination is carried out according to indications for persons with negative serological and skin-allergic reactions to brucellosis 10-12 months after vaccination. Revaccination is carried out cutaneously using a half dose, which is 1 drop (0.05 ml) and contains 5 × 109 m.k. In order to identify contraindications, the doctor (paramedic) on the day of vaccination conducts a survey and examination of the vaccinated person with mandatory thermometry. At temperatures above 37 °C, vaccination is postponed. If necessary, carry out the necessary laboratory examination. Before each vaccination, the vaccine recipient is required to determine the presence of specific immunity using one of the serological or skin-allergic reactions. Persons with a negative reaction to brucellosis are subject to vaccination.
Vaccinations should be carried out no later than 3-4 weeks before starting work associated with the risk of infection.
The vaccination performed is registered in the established registration forms, indicating the name of the drug, date of vaccination, dose, name of the manufacturer of the drug, batch number, reaction to the vaccine.
Skin vaccination
The drug is dissolved with sodium chloride solvent for the preparation of dosage forms for injection 0.9%, which is added to the ampoule with a sterile syringe with a needle at the rate of 0.1 ml of solvent per one vaccination dose. The ampoule is shaken until a homogeneous suspension is formed. The dissolution time of the vaccine should not exceed 1 minute. The reconstituted preparation must be a homogeneous, cloudy suspension of white or white with a yellowish tint, without foreign impurities, sediment or flakes.
The grafting site (the outer surface of the middle third of the shoulder) is treated with alcohol or a mixture of alcohol and ether; the use of other disinfectants is not permitted. After the alcohol and ether have evaporated, without touching the skin, apply two drops of the reconstituted vaccine at a distance of 30-40 mm from each other, stretch the skin and use a sterile scarifier to make 6 cuts (3 longitudinal and 3 transverse), each 10 mm long, through each applied drop of vaccine with a distance between notches of 3 mm. The notches should not bleed, the blood should only appear as dewdrops.
Using the flat side of the scarifier, rub the vaccine into the incisions for 30 seconds and allow to dry for 5 minutes.
When revaccinating, use half the dose, i.e. 1 drop of vaccine, through which 6 cuts are made.
Subcutaneous vaccination
The vaccination dose of the drug with this method is 25 times less than with cutaneous vaccination; The dilution of the vaccine is carried out at the rate of 12.5 ml of sodium chloride solvent for the preparation of dosage forms for injection 0.9% per dose of the vaccine for cutaneous scarification application (subcutaneous dose - 0.5 ml × 25 = 12.5 ml).
The drug is dissolved in the same way as for cutaneous vaccination, after which the resulting suspension is transferred into a sterile injector bottle into which the required volume of solvent is added (for example, if the ampoule contains 7 cutaneous doses of the vaccine, then the contents should be suspended in 12.5 ml ×7, i.e. in 87.5 ml).
The vaccine is administered with an injector designed for subcutaneous administration (BI-ZM or anti-infective protector PPI-2), according to the Instructions for use of the injector in a volume of 0.5 ml.
Vaccinations are carried out in the area of the outer surface of the shoulder on the border between the upper and middle third. The injection site is treated in the same way as for skin grafting.
Contraindications
The brucellosis vaccine cannot be used in a number of cases, including:
- individual intolerance to vaccine components in humans;
- the patient has symptoms of an acute inflammatory disease or an exacerbation of a chronic form of the disease;
- increased body temperature and signs of catarrhal symptoms in humans;
- immunodeficiency states;
- tumor pathological formations and blood diseases that require radiation or chemotherapy;
- diseases of the skin with damage to the epidermis at the intended site of exposure;
- the patient has a history of allergic reactions to the previous administration of the vaccine;
- period of pregnancy and breastfeeding;
- tendency to bronchospasm and severe variants of bronchial asthma.
Ignoring contraindications to the use of a live vaccine against brucellosis is fraught with consequences and complications of immunization, which are sometimes accompanied by complex pathological conditions that are life-threatening for the patient.
Side effects and complications
According to statistical studies, vaccination against brucellosis can provoke the development of the following adverse reactions:
- swelling and inflammation at the injection site, which is sometimes accompanied by the formation of hard red nodules;
- infiltration of tissues around the site of injection of the vaccine solution with the appearance of unpleasant sensations and discomfort;
- development of headaches, sometimes slight dizziness;
- general weakness several days after vaccination;
- increase in body temperature after the procedure;
- allergic reactions in the form of skin rash and redness with itching;
- Quincke's edema and anaphylactic reaction.
Directions for use and doses
The vaccine is administered to patients intradermally in increasing doses in the area of the joints, shoulders or hips at points at a distance of 40-60 mm from each other; the minimum dose is 2x105 microbial cells, the maximum is 3x108 microbial cells.
Before use, each ampoule of vaccine is carefully inspected. The vaccine cannot be used if the packaging is damaged, the appearance changes (foreign particles), the label is missing or damaged, or after the expiration date.
The ampoule with the vaccine is thoroughly shaken until a homogeneous suspension is obtained, wiped with alcohol, the neck of the ampoule is filed, covered with a sterile swab, and the sawn end is broken off. The vaccine from an opened ampoule must be used immediately.
Vaccine dilution technique. The vaccine is diluted immediately before use with 0.9% sodium chloride solution for injection, which is poured into three sterile vials of 9 ml. Add 1 ml of vaccine to the first bottle using a syringe and mix well, then transfer 1 ml of vaccine from the first bottle to the second bottle, and transfer 1 ml of vaccine from the second bottle to the third bottle. In the first bottle, a dilution of the vaccine is obtained 1:10 with a content of 1 × 108 microbial cells in 1 ml, in the second bottle - 1:100 with a content of 1 × 107 microbial cells in 1 ml, in the third bottle - 1:1000 with a content of 1 × 106 microbial cells in 1 ml.
Using the data presented in the table, it is determined how much of the diluted vaccine contains the required number of microbial cells.
| Drug volume, ml | The number of microbial cells when diluting the vaccine | ||
| 1:10 | 1:100 | 1:1000 | |
| 0,1 | 1×107 | 1×106 | 1×105 |
| 0,2 | 2×107 | 2×106 | 2×105 |
| 0,3 | 3×107 | 3×106 | 3×105 |
| 0,4 | 4×107 | 4×106 | 4×105 |
| 0,5 | 5×107 | 5×106 | 5×105 |
| 0,6 | 6×107 | 6×106 | 6×105 |
| 0,7 | 7×107 | 7×106 | 7×105 |
| 0,8 | 8×107 | 8×106 | 8×105 |
| 0,9 | 9×107 | 9×106 | 9×105 |
| 1,0 | 1×108 | 1×107 | 1×106 |
| 1,5 | 1,5×108 | — | — |
| 2,0 | 2×108 | — | — |
| 2,5 | 2,5×108 | — | — |
Vaccine treatment method.
Treatment with intradermal vaccine can be administered in clinical and outpatient settings.
The dosage and duration of treatment is determined by the attending physician, depending on the form of the brucellosis disease and the individual sensitivity of the patient. I determine the allergic reaction of the body! according to the Burnet test indicators before the start of treatment and the body’s response to the first administration of the minimum dose of the vaccine.
The Burnet test is performed using liquid brucellosis allergen for intradermal use in accordance with the instructions for use of the drug.
Reactivity (allergic reaction) can be: 1) insufficient (areresponsiveness, hyporeactivity) - lack of reaction or the appearance of mild swelling (no more than 2 cm in diameter) at the site of allergen injection and without a response to the vaccine; 2) moderate intensity (normoreactivity) - a positive Burnet test (swelling ranging from 2 to 6 cm in diameter), the appearance of a general reaction of the body to the administration of the vaccine in the form of an increase in body temperature to 37.5 ° C, chilling; 3) strong (hyper-reactivity) - a sharply positive Burnet test (swelling over 6 cm, sometimes accompanied by lymphadenitis and a general reaction of the body), the appearance of a general reaction of the body to the introduction of the vaccine in the form of an increase in body temperature above 37.5 ° C, chills, increased pain in the affected areas.
When determining the dosage of the vaccine, the individual sensitivity of the patient should be taken into account each time.
The approximate treatment regimen for patients with brucellosis with normoreactivity with intracutaneous administration of the vaccine with an interval between injections of 2-3 days is as follows:
1 injection - 2x106 microbial cells in 0.2 ml of vaccine dilution 1:100 at 2 points;
2nd injection - 4x106 microbial cells in 0.4 ml of vaccine dilution 1:100 at 4 points;
3rd injection - 1x107 microbial cells in 1.0 ml of vaccine dilution 1:100 at 10 points;
4th injection - 2x107 microbial cells in 0.2 ml of vaccine dilution 1:10 at 4 points;
5th injection - 1x108 microbial cells in 1.0 ml of vaccine dilution 1:10 at 10 points;
6 injection - 2x108 microbial cells in 2.0 ml of vaccine dilution 1:10 at 10 points;
7th injection - 3x108 microbial cells in 0.3 ml of undiluted vaccine at 6 points.
In case of hyperreactivity, treatment begins with a dose of 2 × 105 microbial cells; in case of a pronounced local reaction, the dose of the vaccine should not be increased during subsequent injections.
In case of hyporeactivity, treatment begins with a dose of 1×107 microbial cells.
Repeated courses of vaccine therapy, if necessary, can be carried out no earlier than after 2-3 months.
Video on the topic
About methods of combating brucellosis in the video:
The vaccine against brucellosis is one of the immune drugs, the administration of which is rarely accompanied by pathological reactions and complications. Today it is offered to be done annually for patients from high-risk groups for infection.
Despite the positive qualities of the vaccine, scientists continue to work towards creating an inactivated drug against the brucellosis pathogen, which they first propose to use for revaccination.