Vaccine options
Pertussis vaccine is usually produced in combination with diphtheria and tetanus toxoids (DPT).
The domestically produced vaccine contains a more reactogenic, but also more immunogenic whole-cell pertussis component. In addition, domestically produced combination drugs “Bubo-M”, “Bubo-Kok” and “DTP-HepB” are produced, as well as imported vaccines “Pentaxim”, “Infanrix Penta”, “Infanrix Hexa”, “Tetraxim”, “Infanrix” " Imported AaDPT vaccines (Adsorbed acellular Pertussis-Diphtheria-Tetanus vaccines) contain an acellular pertussis component, which is better tolerated than whole cell, but has lower immunogenicity. Imported vaccines, as well as, for example, the BUBO-Kok vaccine, are often combined with vaccines against hepatitis B, polio (inactivated) and Haemophilus influenzae type b. For age-related revaccinations against pertussis in children over 6 years of age and adults, as a rule, combination vaccines with an acellular pertussis component are used, which also include a reduced content of diphtheria toxoid and tetanus toxoid (Adasel vaccine).
What type of vaccine is there for whooping cough?
The whole cell vaccine contains live bacteria, but they are artificially weakened. Split vaccines contain fragments of pertussis bacillus. Split vaccines do not produce as strong a reaction, but are more expensive. Therefore, the state provides its citizens with budgetary whole-cell vaccinations.
The vaccine is called DPT, which stands for “adsorbed pertussis-diphtheria-tetanus.” 1 ml of vaccine contains 20 billion pertussis bacilli, which is equivalent to 2 doses.
Analogues of whole cell vaccines:
- Tritanrix, which contains components that protect against tetanus, diphtheria, whooping cough, hepatitis B and Haemophilus influenzae.
- Bubo-kok, which is supplemented only with a component that protects against hepatitis B.
Split vaccines:
- Infanrix is an analogue of DTP, containing exactly the same components.
- Infanrix Hexa, in addition to components against whooping cough, diphtheria and tetanus, it contains antibodies to Haemophilus influenzae, polio and hepatitis B. The Hexavak vaccine has a similar effect.
- Pentavac provides protection against diphtheria, whooping cough, tetanus, polio and Haemophilus influenzae.
In Europe, a monocomponent whooping cough vaccine is also used, but in Russia it is not used.
Principles and purposes of vaccination
The main purpose of vaccination against whooping cough is to reduce the risk of acute whooping cough in infancy. It is important to note that children in the age group from 7 to 14 years occupy a leading place in the structure of cases due to the weakening of post-vaccination immunity just by the age of 6–7 years. In school-age children and adults, whooping cough can also be very severe, therefore, to prolong immunity, it is possible to carry out age-related revaccinations at older ages. Moreover, it is school-age children, as well as parents, who are one of the main sources of infection for unvaccinated children in the first months of life, in whom whooping cough is especially severe.
Is it necessary to get a whooping cough vaccine?
The bacterium that provokes the disease quickly loses its viability in external conditions, but when infection does occur, it is almost impossible to get rid of the disease. The pertussis stick lives on surrounding objects for only a few minutes. It can be killed by factors such as sunlight, room disinfection, and even regular cleaning without the use of any specialized products.
Whooping cough is an infectious disease that has an acute course and is accompanied by paroxysmal cough. The causative agent of whooping cough is the Bordet-Gengou bacillus (bordetella). The source of the spread of infection is a sick person. The disease is transmitted by airborne droplets.
The incubation period of the disease lasts 3-15 days and is not accompanied by any symptoms. The catarrhal period is extended for 14 days. At this time, the patient develops a dry cough, body temperature rises to 38 °C, and there is a slight runny nose.
The next period is called “spasmodic”. Its duration is 4 weeks. At this time, the patient suffers from paroxysmal coughing, which is accompanied by short-term cessations of breathing and often ends with vomiting.
The last period of the disease is called the resolution stage. It lasts about 2 weeks. At this time, coughing attacks begin to gradually fade away.
The reasons for getting a whooping cough vaccine are as follows:
- The disease has a long course and is accompanied by paroxysmal cough, which cannot be eliminated by taking medications.
- The cough is characterized by recurrences. During coughing impulses that follow one after another, the child tries to inhale through a narrowed glottis. It ends up making a whistling sound.
- Immunity is not transferred from woman to child at birth, even if she once suffered from the disease.
- To date, no medicine has been found that would relieve the patient’s paroxysmal cough. Treatment comes down to symptomatic therapy.
- Children under the age of one year suffer the disease extremely hard. This age group has an extremely high risk of death.
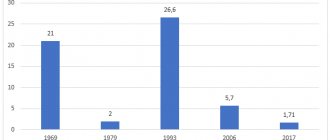
- Before the start of the mass vaccination campaign, epidemics of whooping cough among children were registered in the USSR. Moreover, most often the disease affected children under 5 years of age.
- Thanks to the creation of the whooping cough vaccine, child mortality was reduced by 45 times.
- Once an infection has occurred, it does not provide lifelong immunity.
- Whooping cough is dangerous due to its complications, including rupture of the eardrum, pneumonia, hemorrhage in the conjunctiva of the eye, the formation of an umbilical hernia, and rectal prolapse.
Vaccination also does not guarantee that a person will not get whooping cough. However, if infected, the disease will have a mild course and will not cause serious complications. It costs the state much more to treat whooping cough than to conduct a mass vaccination campaign.
Vaccine effectiveness
Despite significant differences in content and method of manufacture between whole-cell and acellular pertussis vaccines, comprehensive clinical trials have shown that the most effective vaccines of either category protect more than 85% of vaccinated individuals from clinical manifestations of the disease. The best acellular vaccines are as effective as the best whole cell vaccines (85%).
There is still debate about whether monovalent or bivalent acellular vaccines (containing inactivated pertussis toxin alone or in combination with FHA) are as effective as multivalent acellular vaccines (containing 3-5 components). However, all licensed acellular vaccines have been shown to be highly effective in controlling pertussis in infants and young children, provided adequate vaccine coverage (>90%) is achieved.
In 2008, approximately 82% of all infants worldwide were vaccinated with three doses of pertussis vaccine. WHO estimates that approximately 687,000 deaths were prevented by pertussis vaccination in 2008.
Post-vaccination reactions
If vaccinations are carried out with DTP vaccines, then it should be remembered that these vaccines are the most reactogenic, “heavy” vaccine preparations. On average, adverse events occur in a third of vaccinated people. They are manifested by a moderate increase in body temperature and mild malaise within 24 hours after vaccination. As a rule, all post-vaccination reactions to DTP vaccines develop no later than 72 hours after vaccination and last no more than 48 hours. Acellular vaccines are considered less reactogenic. The most characteristic features of acellular vaccines are the low incidence of temperature reactions, local reactions at the injection site and, most importantly, the incidence of post-vaccination complications.
Risk of post-vaccination complications
Possible specific complications of DTP vaccines include neurological complications, which are extremely rare. It is believed that they may be caused by the fact that the toxins (even inactivated) of pertussis bacillus in combination vaccines tend to irritate the meninges of a very small proportion of susceptible children. Complications in the form of encephalopathy are less than 1 case per 300 thousand vaccinated people.
Currently, in the world, convulsions without fever are not considered a complication of vaccination. Research conducted in Great Britain in 1960-1970. indicate the same frequency of seizures in both vaccinated and unvaccinated children. Moreover, the first manifestations of diseases such as epilepsy and organic brain damage can appear at the age of 3-4 months in the form of seizures, when vaccination is carried out, and are associated with vaccination only by a temporary factor.
The development of afebrile seizures indicates the presence of an organic lesion of the nervous system in the child, which was not taken into account and identified or could not be identified before vaccination for objective reasons. Therefore, in the event of afebrile seizures, a comprehensive neurological examination is necessary to make a diagnosis.
Possible complications
In the Russian Federation, children under 6 years of age are given a complex vaccine called DTP. In addition to the pertussis component, it contains cells that protect the child’s body from tetanus and diphtheria.
Vaccines can be live, but inactivated. They are also called whole cell vaccines, but there are also split vaccines consisting of fragments of pertussis bacillus. In our country, inactivated vaccines are used, which have their advantages and disadvantages.
It should be noted that DPT is one of the vaccines that most often causes negative reactions in the body. They are due to the presence of the pertussis component in it.
Local side effects include:
- Hyperemia of the skin at the site of vaccine administration.
- The appearance of a painful lump under the skin. Normally, the seal should not be more than 2.5 cm in diameter.
General reactions of the body can be noted as follows:
- Increased body temperature, which can sometimes reach 38.5 °C.
- General malaise and weakness.
As a rule, these reactions go away on their own after a few hours. However, if a child’s temperature persists for a long time, or the lump exceeds 2.5 cm in diameter, it is necessary to consult a doctor.
The following vaccination complications require taking medications:
- Formation of aseptic infiltrate.
- The appearance of rashes at the site of vaccine administration. A possible allergic reaction to vaccination is angioedema and anaphylactic shock.
- Negative reactions from the nervous system are also possible, which are manifested by loud crying, increased anxiety, and sleep disturbances.
- An encephalopathic reaction may occur to the administration of the vaccine, which manifests itself 2-3 days after the vaccination. It is expressed by convulsions, increased body temperature, and loss of consciousness. If the reaction is weak, the child may experience muscle twitching and tremors of the limbs.
- An extremely rare complication is encephalitis.
- If a child has been given a low-quality vaccine or has a weak immune system, this risks developing post-vaccination whooping cough.
Consultation with a specialist, as well as a high-quality examination of the child before and after vaccination will help minimize the risk of complications. Parents should make sure in advance that there is a drug in the house that will reduce the baby’s temperature.
When to vaccinate?
From 3 months. Vaccination is carried out three times with an interval of 45 days and a single revaccination 12 months after the 3rd vaccination, i.e. at 18 months of age.
The optimal strategy for controlling pertussis infection is the maximum timely coverage of preventive vaccinations for children in the first two years of life within the time frame recommended by the national calendar of preventive vaccinations (at 3-4.5-6-18 months); catch-up immunization of children not vaccinated in a timely manner; carrying out age-related revaccinations against whooping cough in children aged 6-7 years, 14 years old, adolescents and adults from 18 years old every 10 years from the date of the last revaccination.
Timing of vaccination. Where is the whooping cough vaccine administered?
The whooping cough vaccine is included in the routine vaccination schedule. The vaccine is one of the first to be administered to a child, since whooping cough is the most dangerous disease for children under one year of age. If a child becomes infected before the vaccine is administered, he will be urgently hospitalized.
The timing of vaccination against whooping cough is as follows:
- Three months – the first vaccination in combination with tetanus and diphtheria vaccines (DTP).
- 4.5 months – delivery of the second vaccine.
- Six months – production of the third vaccine. The vaccine cannot be administered earlier than 1 month after the previous vaccination.
- At 18 months, children are recommended to receive revaccination, which helps form more stable immunity.
The whooping cough vaccine is administered intramuscularly into the anterior outer thigh. The dose is 0.5 ml.