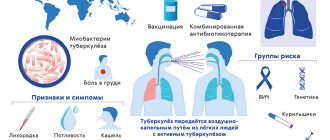
Russian vaccines
Today in Russia only domestic drugs are used to prevent coronavirus disease and its complications. These are the following types of vaccines:
- Vector. Genetically engineered vaccines, where the vector is a virus that is safe for humans. The gene for one of the coronavirus proteins (usually the S protein) is inserted into it, against which antibodies and cellular immunity are produced. Russian vector vaccines include Sputnik V and Sputnik Light.
- Peptide. In this case, ready-made purified coronavirus proteins are used. The Russian "EpiVacCorona" is a chemically synthesized peptide component of the coronavirus S protein.
- Whole virion. Vaccines with weakened or inactivated (killed) viral particles. Whole-virion vaccines include CoviVac.
Russian vaccines: what to choose?
“In Russia, only Sputnik V should be chosen as the first vaccine.” It is the only vaccine with published data and proven efficacy and safety. The effectiveness of CoviVac is unclear. There are no publications. According to the results of groups of volunteers, very few antibodies are produced, and this is an indicator of the degree of immune activation. EpiVacCorona is a failed vaccine. After it, neutralizing antibodies are not formed. An article on the effectiveness of the vaccine, which Rospotrebnadzor published after vaccinating its employees, showed that people were seriously ill, and more often than the average for this period in Moscow. That is, EpiVacCorona did not provide protection.”
Dudnakova Tatyana Valerievna
expert
Research Fellow at the University of Edinburgh, UK
"Gam-COVID-Vac" ("Sputnik V")
Vaccine type: vector
Date of registration in the Russian Federation: August 11, 2020
Vaccination schedule: 2 doses with an interval of 21 days
Efficiency: 91.6%
Trial stage: phases 3 and 4 (interim research results are expected in the fall of 2021)
“Gam-COVID-Vac” (“Sputnik V”) is a combined vector vaccine developed by the National Research Center for Epidemiology and Microbiology named after N.F. Gamaleya. Due to the current extraordinary situation, the vaccine had to be registered after phase II trials, which had not happened before¹.
Phases of drug research
Usually, it takes from 5 to 15 years or more to find a vaccine for a specific pathogen (virus or bacteria).
However, new generation vaccines can be created much faster. For example, if scientists take an already tested and studied vector basis for a vaccine, as was the case with the developers of Sputnik V. In this case, it takes only about two weeks to insert into it the gene of one of the proteins of the infectious agent, to which immunity will then be developed. The longest-term clinical trials, in which the immunogenicity, safety and effectiveness of the vaccine are studied in several stages. In the first phase of the study, the drug is administered to 10-30 volunteers; on the second – 50-500; in the third phase, more than 1000 people of different ages take part.
Assessing the effectiveness and safety of a study drug takes from six months to several years, since both the short-term and long-term effects of the vaccine must be determined. In particular, how long does it protect a person from the pathogen, and are there any side effects.
After registration and start of use of the drug, phase IV research begins. The developer undertakes to collect new information about the effects of the drug (for example, rare side effects) after entering the market. This applies not only to vaccines, but to all drugs.
The vector for Sputnik V is a human adenovirus, into the genome of which the gene for a fragment of the coronavirus S protein is inserted. It is noteworthy that the vectors for the first and second doses of Sputnik V are different. The first component is made based on adenovirus serotype 26, and the second - on adenovirus serotype 5. This approach is not accidental. The fact is that after vaccination, immunity is developed both against the coronavirus S-protein and against the envelope proteins of the adenovirus (vector). This means that repeated introduction of the same vector will be ineffective, since the immune system will quickly destroy it. Therefore, the second component of Sputnik V is based on a different vector.
In November 2021, the first interim analysis of data from the third phase of clinical trials in Russia was published. The declared effectiveness of the Sputnik V drug was 92%. Later, in February 2021, the full interim results of the third phase were published in The Lancet, according to which the effectiveness of Sputnik V is 91.6% after two doses².
Vaccines "Gam-COVID-Vac" and "Sputnik Light". Photos of Maxim Mishin from the website of the mayor and government of Moscow: https://www.mos.ru/mayor/media/photo/8049057/tiles/2/15/ and https://www.mos.ru/news/item/92820073 /
"Sputnik Light"
Vaccine type: vector
Registration date in the Russian Federation: May 6, 2021
Vaccination schedule: 1 dose
Efficiency: 79.4%
Trial stage: phases 3 and 4 (interim research results are expected in the fall of 2021)
Sputnik Light is the first component of the Sputnik V vaccine. The effectiveness of the drug is 79.4% (after one dose). This is a kind of express vaccination. The idea of a single-component vaccine is to cover as many people as possible, giving them at least some protection against Covid.
In practice, it turned out that not everyone in Russia wants to get vaccinated. There is no shortage of vaccines in the country. Therefore, some experts believe that Sputnik Light may be useful for revaccination (the third dose of the vaccine to strengthen immunity).
Advantages and disadvantages of vector vaccines
Vector vaccines such as Sputnik V and Sputnik Light have their own strengths and weaknesses. In theory, they should work as effectively as live vaccines (based on weakened viruses).
The problem with live vaccines is that no one knows how the virus mutates during the production stage of such a drug. There is always the possibility that the weakened virus will be able to regain its former strength, and then the vaccine will turn into a real infection. With vector vaccines there is no such danger, since modified viruses that are safe for humans and do not mutate are used as vectors.
One of the prejudices against vector vaccines is their supposed lack of research. However, it is not. Adenoviruses began to be studied for medical use more than 50 years ago. The first were live vaccines against adenovirus infections, versions of which are still used in the US Army. But it quickly became clear that an adenovirus, deprived of the ability to replicate, is a convenient vector for delivering genetic constructs into cells. Since adenoviruses cannot integrate into host DNA cells, their use as delivery vectors is safe and effective. Adenoviruses are also used in oncology therapy.
Adenoviruses are most widely used as the basis for coronavirus vaccines. For example, the AZD1222 vaccine based on the chimpanzee adenovirus, developed by scientists from Oxford, is widely used today in the world. It is slightly less effective than the Russian Sputnik V.
The disadvantage of vector vaccines is the fact that in addition to the response to the spike protein, immunity to the adenovirus envelope proteins is developed. Immunity to adenoviruses is unstable, but it is not yet clear how many times such vaccination can be repeated. In the meantime, from the UK’s experience with the AstraZeneca vaccine and the experience of groups of Sputnik V volunteers, we know that booster vaccination with an adenovirus vaccine is effective if done at least 3 months after vaccination. However, it is unknown how many times it can be repeated. At the same time, it is clear that Covid vaccinations may become an annual routine.
"CoviVac"
Vaccine type: whole virion inactivated
Registration date in the Russian Federation: February 19, 2021
Vaccination schedule: 2 doses with an interval of 2 weeks
Efficacy: not established (still at the research stage)
Trial stage: phase 3 (interim trial results expected December 30, 2021)
An inactivated vaccine against the new coronavirus was created at the Federal Scientific Center for Research and Development of Immunobiological Preparations named after. M. P. Chumakova. By the end of 2020, it was possible to complete all preclinical studies (on cell cultures and animals) and begin the clinical phase of testing.
The virus for the production of the CoviVac vaccine was isolated from a patient who was treated at City Clinical Hospital No. 40 (Kommunarka). The isolated virus was then cultivated (propagated) in cell cultures and killed with a chemical reagent - beta-propiolactone.
It is planned to produce up to 10 million doses of CoviVac annually. However, phase 3 clinical trials are still ongoing. 3,000 volunteers take part in it. The study is planned to be completed by the end of 2021, after which it will become clear how effective and safe this vaccine is.
Advantages and disadvantages of inactivated vaccines
The advantage of inactivated vaccines over live ones is that there is no risk of infection. Killed viruses cannot mutate, and therefore there is no potential danger in them.
- The advantage over vector and mRNA vaccines is that the whole virus is injected into the patient. This leads to the formation of immunity against all viral proteins, and not just a fragment of the S protein. Previously, it was believed that the S protein was conserved and would not change in mutated strains. But in practice we see something different. This results in reduced effectiveness of both vector and mRNA vaccines that target the S protein alone.
- Inactivated vaccines also have disadvantages. They form incomplete antiviral immunity. This is due to the fact that they are not able to penetrate the cell (and viruses are intracellular parasites) and cannot form a T-cell immune response. Therefore, adjuvants are used to enhance their effectiveness. The CoviVac vaccine uses aluminum hydroxide.
- In addition, during chemical treatment, some proteins lose their structure, including the spike protein, resulting in the loss of many epitopes. In groups of volunteers, it was noticed that after CoviVac, significantly fewer antibodies to the spike protein were produced, namely, neutralizing antibodies that protect against infection. Theoretically, inactivated vaccines have an increased risk of autoimmune reactions, but these concerns with CoviVac have not yet come true. Serious disadvantages of whole-virion vaccines are the need to increase large volumes of a dangerous pathogen and the difficulty of scaling vaccine production, when chemical exposure conditions must be selected individually for each batch. As a result, they decided not to put into production a similar vaccine being developed in Scotland. The main disadvantage of inactivated coronavirus vaccines (we can see this in the example of Chinese analogues) is their lower effectiveness compared to vector and RNA vaccines.
"EpiVacCorona"
Vaccine type: peptide
Registration date in the Russian Federation: May 6, 2021
Vaccination schedule: 2 doses with an interval of 14-21 days
Efficacy: not established
Test stage: phase 3 (disclosure of research results is planned in the summer of 2021)
“EpiVacCorona” is a development of the State Scientific Center for Virology and Biotechnology “Vector”. The vaccine is a suspension containing three small protein fragments (peptides) of the coronavirus S-protein.
EpiVacCorona began to be used before the end of the second phase of clinical trials3. The third phase began at the end of 2021 and continues to this day. The results of the research are planned to be announced in the fall of 2021 (the deadlines have been repeatedly postponed by the developer).
As with inactivated vaccines, peptide vaccines also contain an adjuvant (aluminum hydroxide), a substance that enhances the immune response.
As for the effectiveness of EpiVacCorona, the question remains open. Interim results of the second phase of research were published in the Russian journal Infection and Immunity, where the drug was declared 100% effective. However, critics have great doubts about this, since the impact factor (citation rate) of this journal is only 0.68. For comparison, the IF of The Lancet (which published data on Sputnik V) is 60.4, which is almost 100 times more.
Advantages and disadvantages of peptide vaccines
The advantage of protein vaccines is that they can be tested quickly. They are considered safer than other vaccinations.
The problem with protein vaccines is the difficulty of producing peptides. In addition, such drugs are not highly effective. Most likely, immunity from peptide vaccines will be unstable and the patient will need revaccination.
Our immune system and active ingredients
The active ingredient in a vaccine is usually made from a viral or bacterial pathogen. There are two different approaches to this, with the pathogen either alive or inactivated. Vaccines containing live bacteria or viruses are called live attenuated vaccines
. The pathogen is weakened, but is still capable of causing a strong immune response.
Live attenuated vaccines are not suitable for everyone. If a person has a weakened immune system, they may get the disease that the vaccine is supposed to protect them from.
Because of this, more often vaccines use an inactivated version of the active ingredients, taking the form of whole bacteria or viruses that have been killed. However, most vaccines are actually acellular
, which means they do not contain the entire pathogenic organism. Instead, they are made up of parts of the pathogen, such as proteins or sugar molecules. Our body recognizes these molecules as foreign and produces an immune response.
Examples of acellular vaccines are:
- toxoid vaccines containing inactivated toxins from pathogenic bacteria
- Conjugate vaccines are made from a combination of pathogen-specific sugar molecules and toxoid proteins, since the sugars themselves do not cause strong enough immune responses
- recombinant vaccines made by using bacteria or yeast cells to make many copies of certain molecules from the pathogen
Foreign vaccines
Foreign vaccines against coronavirus.
Photo: marcbruxelle / Depositphotos The most popular foreign vaccines are mRNA drugs from Pfizer/BioNTech and Moderna, a vector vaccine from AstraZeneca, as well as Chinese whole-virion drugs from Sinovac and Sinopharm.
Pfizer/BioNTech and Moderna are the world's first mRNA vaccines
Vaccine developers from Pfizer/BioNTech and Moderna have decided to take a different route in solving problems with coronavirus. The fundamental difference between mRNA vaccines is that viral proteins are synthesized in the human body. Messenger RNA (mRNA) is a kind of instruction, after reading which the cell begins to produce the encoded protein4. In this case, it is a fragment of the coronavirus S protein.
Today, mRNA vaccines against coronavirus are used in the USA, Great Britain, the European Union, Israel, Ukraine and many other countries. Preliminary results show good protection against hospitalizations and deaths. In particular, in the USA (where more than 55% of the population has been vaccinated) they are already talking about an epidemic of unvaccinated people. The vast majority of patients admitted to hospitals with Covid are people who have not been vaccinated.
Advantages and disadvantages of mRNA vaccines
Messenger RNA vaccines are highly effective because they penetrate cells. Imitating infection with a virus leads to the formation of full immunity. These drugs do not require adjuvants. In addition, mRNA vaccines can be developed quickly. If live or inactivated vaccines need to be grown in cell cultures, then mRNA drugs are “printed” on special synthesizers. In emergency situations, when it is necessary to vaccinate a large number of people in a short time, this is a rational approach.
The disadvantage of mRNA drugs is that they are not well studied. These are new vaccines that have never been used before. There is no data yet on the long-term effects of such vaccinations on human health.
Vector vaccine from AstraZeneca
This is a vaccine made according to the same principle as Sputnik V. In this case, a modified chimpanzee virus5 is used as a vector.
The AstraZeneca vector vaccine is 79% effective against symptomatic and 100% effective against severe COVID-19. This is the main vaccine used in EU countries. In the spring of 2021, some states temporarily suspended vaccination with this drug due to data on thrombosis and other serious consequences. However, European regulators soon allowed the vaccine again.
Figure 1. Types of COVID-19 vaccines. Sources: Rospotrebnadzor and WHO (WHO): https://www.rospotrebnadzor.ru/about/info/news/news_details.php?ELEMENT_ID=15468, https://www.who.int/ru/news-room/feature -stories/detail/the-race-for-a-covid-19-vaccine-explained
Whole virion vaccines from Sinopharm and Sinovac
Chinese biopharmaceutical companies Sinopharm and Sinovac have become major suppliers of coronavirus vaccines. They developed whole-virion anti-Covid vaccines similar to CoviVaca.
In addition to China, these vaccines are actively used in Turkey, Chile, the United Arab Emirates, Argentina and other countries. The results of the third phase of studies in different countries range from 50 to 84%6. Moreover, the vaccines are 100% effective in preventing severe forms and death.
Wrong? Admit it!
“Developing vaccines is difficult.
Failures are normal, the main thing is to admit them. EpiVacCorona does not work and cannot be injected, but it is available for vaccination. There have been several vaccines around the world that don't work. For example, SanoFi Institute Pasteur is a protein vaccine, somewhat similar to EpiVacCorona. It contains a spike protein expressed in the baculovirus system. The Germans tried to make an mRNA vaccine and it didn't work. An inactivated whole-virion vaccine was developed in Scotland, but it was decided not to put it into circulation. Inactivated vaccines are less effective against covid than gene-transferring ones. Chinese whole-virion inactivated vaccines have not shown themselves to be very effective; their effectiveness is low. Seychelles and Chile, which vaccinated the population with these vaccines, received a wave of Covid with hospitalizations and deaths and were forced to urgently re-vaccinate the population with the AstraZeneca vector vaccine.
The vector and mRNA vaccines used today are highly effective, leaving the inactivated variants behind by a wide margin. In terms of immunogenicity and effectiveness, the vaccines go like this: Pfizer/BioNTech, Moderna, then Sputnik V and AstraZeneca.”
Dudnakova Tatyana Valerievna
expert
Research Fellow at the University of Edinburgh, UK
Fast does not mean bad: why was the coronavirus vaccine developed in a short time?
The development and testing of the vaccine took about a year. This is a unique, but understandable, case. First of all, the creation of Sputnik V was accelerated by the fact that the vaccine was made on the basis of an adenoviral vector. This technology has long been known to scientists, and the center itself. Gamaleyi had experience in producing similar vaccines. A vaccine against Middle East respiratory syndrome was previously developed. The virus that causes it, MERS, also belongs to the group of coronaviruses.
The rapid spread and ability of the virus to mutate required drastic decisions. At the same time, the safety and effectiveness of the vaccine comes first. That is why the first phases of the study were carried out in compliance with all international rules: the number of volunteers in each phase, the criteria for assessing their health status after vaccination. Only after the vaccine showed its safety and ability to protect against coronavirus was the decision made on mass vaccination.
Comparison of Russian vaccines
For convenience, Table 1 provides comparative characteristics of Russian vaccines against the new coronavirus.
Table 1. Comparison of Russian vaccines against the SARS-CoV-2 virus.
| Name | Composition of the drug | Storage | Immunogenicity | Efficiency | Adverse reactions | Method and scheme |
| "Sputnik V" ("Gam-COVID-Vac") | Recombinant adenoviruses 26 and 5 serotypes with the gene for a fragment of the coronavirus S-protein, salts for stabilizing the drug in solution | At a temperature not higher than minus 18 ℃. Storage in liquid form is allowed at a temperature of 2-8 ℃ | Cellular and humoral immunity is formed on the 42nd day after vaccination. According to research, neutralizing antibodies are formed in 98% of people | Overall efficiency – 91.6%. For persons over 60 years old – 91.8% | Pain at the injection site. Flu and cold-like symptoms. Muscle and joint pain, malaise, headache | Injectable vaccine. 2 doses 21 days apart |
| "Sputnik Light" | Recombinant adenovirus serotype 26 with the gene for a fragment of the coronavirus S-protein, salts for stabilizing the drug in solution | At a temperature not higher than minus 18 ℃. Storage in liquid form is allowed at a temperature of 2-8 ℃ | Neutralizing antibodies are detected in 96.9% of people on the 28th day after vaccination | Declared effectiveness – 79.4% after 1 dose | Pain at the injection site. Flu and cold-like symptoms. Muscle and joint pain, malaise, headache | Injectable vaccine. Single administration |
| "CoviVac" | Antigen of inactivated coronavirus SARS-CoV-2, adjuvant, salts to stabilize the drug in solution | At temperature 2-8℃. The vaccine should not be frozen | At the research stage | At the research stage | Injectable vaccine. 2 doses 14 days apart | |
| "EpiVacCorona" | Chemically synthesized coronavirus S-protein peptides, adjuvant, salts to stabilize the drug in solution | At temperature 2-8℃. The vaccine should not be frozen | Presumably, immunity is formed on the 35-40th day after full vaccination. Neutralizing antibodies are formed in 82.1% of people | Not installed. Theoretically, it can be effective against different strains, since it contains conservative peptides of the coronavirus S protein | Pain at the injection site. Short-term increase in temperature to 38-38.5 ℃ | Injectable vaccine. 2 doses 14-21 days apart |
The effectiveness of vaccines against new strains of coronavirus
Data on the effectiveness of existing vaccines (Russian and foreign) are contradictory.
Leading manufacturers of COVID-19 preventive drugs such as Pfizer, Moderna and AstraZeneca report continued high effectiveness of vaccines against the delta strain. We are talking about 90-96% protection from vaccinations against hospitalization and death. According to data from the UK, Sputnik V is also effective against the delta strain (90% effective). As for protection against symptomatic forms of Covid, in Israel the effectiveness of the Pfizer/BioNTech drug was 64%. A little later, the Israeli Ministry of Health said that this figure had dropped to 39%.
Given the likely decrease in the effectiveness of existing drugs against new strains, manufacturers are thinking about creating new vaccines. The technologies will remain the same (vector, mRNA vaccines), but the vaccines will target other components of the virus (which do not change with mutations).
How does a vaccine create acquired immunity?
When you first become infected with an infection, the immune system takes some time to develop acquired immunity. It works more efficiently than the innate one and can protect us throughout our entire lives, but such immunity is not formed immediately.
The same principle applies to vaccination. Simply put, the development of acquired immunity occurs in four stages:
- Induction. Macrophages attack foreign cells and transmit information about the antigen to lymphocytes.
- Immunoregulatory stage. T and B lymphocytes are targeted to fight the infectious agent.
- Effector stage. Specific antibodies and T-lymphocytes are produced against the infection.
- Formation of immunological memory, which allows you to instantly respond to the invasion of the pathogen against which you were vaccinated.
It takes about a month to develop protection, so vaccination against influenza or tick-borne encephalitis is done in advance.
Contraindications to vaccination
The benefits and potential risks of vaccination are assessed by a doctor.
Photo from the website of the mayor and government of Moscow https://www.mos.ru/mayor/themes/18299/6980050/ The list of contraindications is indicated in the instructions for the specific vaccine. Read it carefully and consult your doctor before getting vaccinated. Let's look at the main contraindications to existing Russian vaccines.
The most famous myths about vaccinations
There have always been a lot of rumors surrounding the safety of vaccines, and some of them have given rise to very persistent myths, despite the fact that there is no evidence of their veracity. Interestingly, many people take myths about the dangers of vaccines more seriously than the real threat posed by infections. We have already forgotten why measles, whooping cough and polio are terrible. It seems to us that these diseases will not return, and therefore we begin to fear vaccinations more than diseases.
Do vaccines really cause autism?
This is a common myth. In 1998, the Lancet published a paper on the link between the measles, rubella and mumps vaccine and autism. Later, the data given in it was found unreliable: some of it was falsified by the author of the article, Andrew Wakefield. In 2010, he was stripped of his medical license for violating professional ethics.
Is it true that a vaccine can chip a person?
Chipping (introducing an electronic chip) into people using vaccinations is technically impossible. Often there are several doses of the drug in one bottle. Even if it contains microchips, it is impossible to dose them by drawing the solution with a syringe from the bottle. If the ampoule contains one dose of the vaccine, the chip administered with the drug must be smaller than the diameter of the injection needle. But in this case, it may remain on the walls of the bottle with traces of liquid, which means there is no guarantee that chipping will occur. The production of vaccines is carried out under very strict control, and the entry of any foreign components into the drug is excluded.
"Sputnik V" and "Sputnik Light"
Absolute contraindications
The drugs “Sputnik V” and “Sputnik Light” are contraindicated in the following cases:
- hypersensitivity to any component of the drug (or similar drugs containing the same components);
- history of severe allergies;
- infectious and non-infectious diseases at the acute stage (you can get vaccinated 2-4 weeks after recovery or remission);
- pregnancy and breastfeeding (issue under discussion);
- age up to 18 years (clinical studies for adolescents are underway).
If severe adverse symptoms (for example, a severe allergic reaction) occur after the first dose of the drug, then the second dose is contraindicated. In some cases, the doctor considers re-vaccination with another drug (CoviVac or EpiVacCorona), but not earlier than 3 months after the first dose of Sputnik.
Vaccinate with caution
For some chronic diseases, Sputnik V and Sputnik Light are used only after consulting a doctor who carefully studies the patient’s medical history. Such diseases include:
- chronic liver and kidney diseases;
- diseases of the endocrine system (diabetes mellitus, severe thyroid disorders);
- severe pathologies of the hematopoietic organs;
- epilepsy;
- acute cerebrovascular accident;
- inflammatory heart diseases (myocarditis, endocarditis and pericarditis);
- malignant tumors;
- autoimmune diseases.
In such cases, the doctor’s task is to assess the benefits and potential risks of vaccination.
"CoviVac"
Absolute contraindications
The whole virus vaccine CoviVac cannot be administered under the following circumstances:
- if you have previously had a severe reaction to any of the vaccines (for example, temperature up to 40 ℃, swelling, severe allergic reaction, convulsions);
- if there is a history of severe allergic reactions (anaphylactic shock, Quincke's edema, eczema);
- pregnancy and breastfeeding;
- age up to 18 years.
Vaccinate with caution
CoviVac cannot be used for some acute and chronic diseases at the acute stage. These include:
- acute infections;
- chronic infections in the acute stage;
- chronic pathologies of internal organs (liver, kidneys, heart, lungs, gastrointestinal tract);
- immune disorders.
CoviVac and immunodeficiency
CoviVac is NOT contraindicated for patients with immunodeficiency conditions. However, the effectiveness of the vaccine may be reduced in people taking immunosuppressants (drugs that suppress the immune system). If possible, it is advisable to discontinue such drugs within a month before and after vaccination. The decision on such correction in treatment is made only by the attending physician.
"EpiVacCorona"
Absolute contraindications
The EpiVacCorona peptide vaccine cannot be used in the following cases:
- hypersensitivity to the components of the drug;
- severe allergic diseases;
- acute and chronic (in the acute stage) pathologies;
- primary immunodeficiency (hereditary disease leading to disruption of the immune system);
- malignant tumors;
- blood diseases;
- age up to 18 years.
Vaccinate with caution
For some diseases and conditions, a patient can be vaccinated only after consulting a doctor. Such diseases include:
- liver and kidney diseases;
- endocrine diseases;
- severe pathologies of the central nervous system (epilepsy, stroke and others);
- heart and vascular diseases;
- secondary immunodeficiencies (acquired during life);
- autoimmune diseases;
- mild or moderate allergic reactions.
Development of a vaccine against coronavirus. Photo: Russian Ministry of Defense / YouTube:
Why is vaccination important for adults and children?
The mechanism of active immunization is designed to prevent the mass spread of viral infections - measles, rubella, hepatitis, mumps, as well as bacterial ones - whooping cough, diphtheria, tetanus and many others.
Vaccinations are required for:
- preventing infection, reducing the risk of contracting life-threatening diseases;
- protection against fatal incurable diseases, for example, rabies, human papillomavirus;
- prevention of serious complications, since after vaccination a small risk of getting sick remains, but the disease will be easier, and the likelihood of death is reduced several times;
- creating immunity in the majority of the population (at least 2/3 of the population). When collective immunity is formed, the spread of infection stops and new cases of infection do not appear.
It is necessary to be vaccinated against some infections not only in childhood, but also in adulthood, since over time the immune system weakens and needs additional stimulation. Revaccination of the adult population is important because childhood infections are much more difficult for them and more often cause complications.
Vaccinations for adults are mandatory when moving across regions and when traveling to countries with unfavorable epidemiological conditions.
For people with chronic diseases, vaccination is indicated to prevent complications.
Children need vaccinations to prevent severe infectious diseases that are difficult to treat and cause life-threatening complications.
Side effects
Mild to moderate side effects from vaccines are considered normal. You should not worry if you have a fever or cold or flu symptoms after vaccination. Within a few days, such reactions disappear on their own.
The most common side effects after vaccination against the new coronavirus include:
- pain at the injection site (the whole shoulder may hurt);
- increased body temperature;
- flu-like symptoms;
- headache;
- muscle and joint pain;
- diarrhea (diarrhea).
According to all vaccination rules, after vaccination the patient must remain in the medical facility for another 20-30 minutes. This is necessary in order to provide assistance to the patient if an allergic reaction suddenly develops.
The US, UK and some other countries maintain an open registry of side effects from vaccination. Among the very rare but serious consequences are myocarditis (inflammation of the heart) and thrombosis (blockage of blood vessels with blood clots). No such effects have been recorded from Russian vaccines. Here you need to take into account the fact that Russia does not maintain an open register of side effects.
Regarding the long-term side effects of Covid vaccines, information is collected in real time. Almost a year after the start of large-scale clinical trials, long-term side effects have not yet been established.
Are vaccines and vaccinations the same thing?
Vaccination by definition is the process of administering a vaccine; this term comes from the verb “to inoculate” and is synonymous with vaccination. Vaccinations are given according to a specific schedule, they are noted in the medical record or vaccination certificate. Everyone should follow the vaccination schedule, especially for people who live in close groups. In kindergartens, schools or the army, conditions are perfect for the spread of many serious infections transmitted by airborne droplets or through food and utensils.
Important! When vaccinating against the same disease, different vaccines may be used; for example, children's vaccines are not always suitable for adults. Often one vaccine protects against several diseases at once (like the DTP vaccine against whooping cough, diphtheria and tetanus). This helps reduce the number of injections and trips to the doctor.
Sources
- The Russian Ministry of Health has registered the world's first vaccine against COVID-19. Ministry of Health of the Russian Federation (08/11/2020). Date accessed: August 11, 2021. Archived August 12, 2021.
- Logunov DY et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomized controlled phase 3 trial in Russia (English) // The Lancet. — 2021. — 2 February. — ISSN 0140-6736. - doi:10.1016/S0140-6736(21)00234-8
- Registration certificate and Instructions for medical use of the medicinal product “EpiVacCorona vaccine based on peptide antigens for the prevention of COVID-19” dated 10.13.2020 + Change No. 1 from 01.13.2021 + Change No. 2 from 02.10.2021 + Change No. 3 of 03/03/2021 // Electronic images of documents on the website “State Register of Medicines”.
- Clinical trial number NCT04368728 for “NCT04368728: Study to Describe the Safety, Tolerability, Immunogenicity, and Efficacy of RNA Vaccine Candidates Against COVID-19 in Healthy Individuals” at ClinicalTrials.gov
- University of Oxford. A Phase 2/3 Study to Determine the Efficacy, Safety and Immunogenicity of the Candidate Coronavirus Disease (COVID-19) Vaccine ChAdOx1 nCoV-19. — clinicaltrials.gov, 2020-12-08. - No. NCT04400838.
- Tanriover MD, Doğanay HL, Akova M, Güner HR, Azap A, Akhan S, Köse Ş, Erdinç FŞ, Akalın EH, Tabak ÖF, Pullukçu H, Batum Ö, Şimşek Yavuz S, Turhan Ö, Yıldırmak MT, Köksal İ, Taşova Y, Korten V, Yılmaz G, Çelen MK, Altın S, Çelik İ, Bayındır Y, Karaoğlan İ, Yılmaz A, Özkul A, Gür H, Unal S; CoronaVac Study Group. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomized, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021 Jul 17;398(10296):213-222. doi: 10.1016/S0140-6736(21)01429-X. Epub 2021 Jul 8. PMID: 34246358; PMCID: PMC8266301.
Constructor elements
Figure 3. Design of a DNA vaccine based on the pVAX1 vector with a chimeric gene (Rat cDNA, Human cDNA). Pcmv—cytomegalovirus promoter; MCS—multiple gene cloning site; BGH pA - terminator with a signal for polyadenylation of the bovine growth hormone gene; Kanamycin—kanamycin resistance gene; pUC ori—the origin of replication of pUC group plasmids; HindIII, BstEII, XbaI - restriction sites. Figure from [5].
To be useful for creating DNA vaccines, every self-respecting vector must contain the necessary structural elements (Fig. 3).
- Structures ensuring plasmid replication (ori pUC19, pMB1 are used) and restriction sites.
- Selective markers: genes for resistance to antibiotics (but not to penicillin and other β-lactam antibiotics) [5].
- CpG motifs of bacteria, which, due to the lack of methylation, are able to enhance the immune response. This principle underlies the development of a universal vaccine and implies the use not of genes encoding microbial antigen proteins, but of bacterial CG sequences as the active component of the vaccine [7].
- The Kozak sequence is a consensus sequence surrounding the start codon (GCC(A/G)CC AUG G) that plays an important role in translation initiation in eukaryotes.
- A promoter for the expression of a target gene in eukaryotic cells. The most commonly used promoters are the SV40 virus, cytomegalovirus (often together with intron A), the beta-actin promoter, promoters specific to certain tissue types (for example, the desmin gene promoter for expression in myocytes, the vitamin D3 hydroxylase gene promoter in keratinocytes, albumin - in hepatocytes). The use of the promoter and synthesis system of bacteriophage T7 allows expression of the target gene without the participation of the transcriptional system of the cells of the macroorganism and, accordingly, without moving the vector into the nucleus [8].
- Target gene encoding a pathogen protein. It may also contain additional nucleotide sequences encoding ligands for receptors on antigen presenting cells. Such sequences can be the genes of the CD40 marker protein, the extracellular domain of Fms-like tyrosine kinase-3, or T-killer antigen-4. Facilitating antigen degradation in the proteasome or lysosome will also stimulate the immune response. Therefore, to enhance the proteolytic cleavage of the antigen, a ubiquitination signal is inserted into its sequence [9, 10].
- The target gene is followed by polyadenylation signals, for example from the SV40 virus, the rabbit β-globin gene or bovine growth hormone.
- Stop codons close this chain, and double or triple termination sequences (TAGTGATGA) are often used.