Flu is a dangerous viral disease. Long before a possible “encounter” with it and other respiratory viruses, we try to strengthen our immune system and take vitamins. However, there is a much more effective method of dealing with the flu - vaccine prevention. We have prepared up-to-date information about the upcoming 2017-2018 flu season and influenza vaccines.
Unraveling the flu: what do we know?
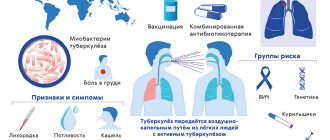
Every year, the disease can be caused by different subtypes/strains, but this does not make it any easier, and sometimes even more severe and unpredictable. What does this have to do with?
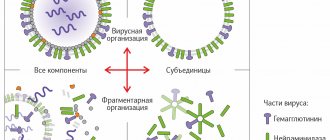
The virus shell contains two proteins that determine the course of the disease:
* Neuraminidase (N) is responsible for the reproduction of the virus and weakens the immune system.
* Hemagglutinin (H) helps the virus attach, damage and penetrate our cells, determines the severity of the disease and the severity of intoxication (fever, feeling unwell, etc.).
The elusive “shape” of the virus
It would seem that after suffering from the flu there is no longer any fear of re-infection. However, the virus constantly and quickly changes/mutates, so the next time it is encountered, the immune system “does not recognize” it and infection occurs again.
The emergence of new and dangerous subtypes of viruses is due to:
* Various combinations of neuraminidase and hemagglutinin - variants arise, A (N1H1), A (H3N2), etc.
* The ability of the human influenza virus to “mix” with other respiratory viruses or animal influenza viruses. This is how hybrids are formed - for example, “swine” flu or “bird” flu.
On a note
* The most variable and dangerous influenza virus is type A, often leading to the development of a large number of complications or even death.
* Type B is less variable and less severe, but also causes considerable harm to health.
Learn more about the differences between influenza and ARVI - in the form of a convenient table
Who is recommended to get the flu vaccine?
Doctors strongly recommend getting vaccinated against influenza:
- children over 6 months of age and older people over 60 years of age, as they are at risk of severe influenza due to their age;
- people with weak immunity, as well as chronic diseases of the cardiovascular system, lungs and kidneys to protect against possible complications of influenza;
- pregnant women, since the flu can negatively affect the development of the fetus and the health of the woman herself.
Immunity and flu
After encountering the virus, antibodies (protective blood proteins) are produced specifically against the type of influenza that led to the development of the disease. Immune memory lasts for approximately three years.
It is believed that small and partial protection against other types of influenza virus - cross immunity - can be formed at the same time.
2017—2018 What kind of influenza virus is expected to invade?
The outbreak is seasonal: usually in January-February. For this season, the incidence of influenza B is predicted - Brisbane, influenza A (H3N2) - Hong Kong, A (H1N1) - Michigan (the most dangerous).
The annual forecast is compiled by WHO in March based on data received on the disease from regions where the influenza season has already begun: countries of Asia, Australia, South America.
Is the vaccine effective against all types of cold and flu viruses?
Seasonal flu vaccines are designed to protect against infections and diseases caused by the three or four flu viruses (depending on the vaccine) that research shows will be most common during the flu season. “Trivalent” influenza vaccines are designed to protect against three influenza viruses, while “tedrivalent” influenza vaccines protect against four influenza viruses. Flu vaccines do not protect against infections and illnesses caused by other viruses, which can also cause flu symptoms . In addition to influenza viruses, there are many other viruses that can cause influenza-like illness (also known as influenza-like illness or “ILI”) that spreads during the flu season. These non-flu viruses include rhinovirus (one cause of the “cold”) and respiratory syncytial virus, which is the most common cause of severe respiratory illness in young children and the leading cause of severe respiratory illness in adults age 65 and older.
Vaccination: everything should be in season, safe and effective
The rapid mutation of the influenza virus means that the previous year's vaccines are in most cases ineffective against the current year's strains. Therefore, new vaccine preparations are developed every year.
Production stages
The virus discovered and isolated from a person is grown on special media. The resulting pathogen is then purified and divided into small pieces - antigens, to which our immune system reacts.
When making a standard vaccine, particles of three types of influenza viruses are mixed in a small concentration, but antigens of four viruses can also be used.
The safety and effectiveness of the resulting drugs are tested first on animals, then on volunteers. Only then are vaccinations widely used.
What is the action based on?
Vaccines mainly contain hemagglutinin and neuraminidase, but sometimes only hemagglutinin. Proteins are isolated from the shell of purified viruses of type A and B of the current year.
It is for meeting them that our immune system must prepare: to develop antibodies (protective proteins) in advance. It has been clinically proven that then the disease either does not develop or proceeds easily and without complications.
Some vaccinations include substances that enhance the effects of vaccines - this can reduce the number of virus particles in the drug and improve the immune response. For example, polyoxidonium or sovidone is used.
Types and routes of administration
* The effectiveness of inactivated vaccines - particles of killed viruses are used. They do not lead to the development of the disease, but contribute to the development of an immune response. The drugs are administered intramuscularly or intradermally.
* The live vaccine is ineffective , so it is not recommended for use.
Vaccinations used
Domestic: “Sovigripp” and “Grippol Plus”, “Microflu”, “Grippovac”, AGH vaccine, “Grifor”, “Ultrix”.
Imported: Agrippal, Fluarix and Begrivak (Germany), Vaxigrip (France), Inflexal V (Switzerland), Influvac (Netherlands).
The vaccine has been introduced. What to expect in the first days?
A local reaction is allowed - slight redness and swelling, general - a moderate and short-term increase in body temperature.
The vaccine cannot cause disease because it contains killed virus particles. If signs of acute respiratory viral infection nevertheless appear, it means that a virus infection has occurred against the background of a somewhat weakened immune system.
Does the flu vaccine protect against SARS?
Indirectly, yes. By improving the functioning of the immune system as a whole. Vaccinations containing substances that enhance the effect of influenza vaccines work especially well in this area.
Who should get vaccinated and who should not risk it?
Does everyone need a vaccine? Strictly speaking, no. Only if the person does not have chronic diseases, is not sick, or easily tolerates viral infections.
Unfortunately, there are not so many healthy people, so in most cases it is recommended to get vaccinated.
Deadlines and general principles
The optimal time for vaccination is before the end of October, so that the immune system has time to develop antibodies before the start of the flu season.
With early administration, the risk of side effects is reduced. After all, the likelihood of getting a cold or acute respiratory viral infection after vaccination is not yet so high at this time of year, so the immune system works without stress, gently and slowly.
What to do if you didn’t have time ? The vaccine is administered until there is a risk of infection with these particular types of virus. True, its effectiveness is already much lower.
Influenza vaccine in combination with other vaccinations
Some of them can be administered simultaneously, depending on the composition, but in different places - the doctor makes the decision individually. If necessary, a 4-week interval is maintained between vaccinations.
Number of doses and regimens used
Children from the age of 6 months to 8 years of age should receive two doses of the vaccine at an interval of 4 weeks by the end of October. If a child was vaccinated with two doses of the vaccine before July 1 of the current year, then in October he will be vaccinated with one dose.
Adults and children over 8 years of age receive only one dose of the vaccine.
Indications - high-risk groups for the disease and the development of complications:
1. Infants (over 6 months) and the first years of life - in case of infection, an imperfect immune system may not cope with the onslaught of the virus.
2. If you are crowded and in a confined space , there is a high probability of infection:
* Children attending kindergarten or from orphanages, school students and boarding school pupils.
* Adults: students, transport and utility workers, teachers, medical workers, residents of a dormitory or nursing home, military personnel and other persons.
3. Adults and children with chronic diseases are at high risk of severe disease and complications. For diabetes, obesity, heart and vascular diseases, chronic kidney and gastrointestinal diseases, disorders of the immune system, HIV infection and others.
5. Persons over 60 years of age - there is a high probability of complications or even death.
Benefits of treating influenza with herbal vaccines
The quadrivalent vaccine with virus-like particles of plant origin was invented not so long ago and has not yet become widely used, but its advantages over other vaccines are already clearly emerging.
No age restrictions
For example, attenuine (live vaccines) are not recommended for use by people over 50 years of age, that is, they cannot get vaccinated once a year to prevent influenza, and to prevent diseases they must constantly take antiviral pills. In a study of people over 65 years of age, the QVLP vaccine [Verified Source] was shown to be effective for use in older adults.
Effective on more viruses
This medical product of biological origin is quadrivalent, which means that its ultimate goal is to develop immunity in the body to 4 types of viruses, two each from group A and B. It should be noted that inactivated and attenuin vaccines are trivalent and can protect only against 2 types of viruses A and one virus from group B.
Does not have foreign impurities
Because QVLP is a plant-derived vaccine [Verified Source] and is unlikely to be contaminated with foreign microorganisms, it is considered purer than trivalent flu medications.
Low percentage of adverse reactions
For people aged 18 to 64 years, the chance of experiencing side effects is 1%, and from age 65 it increases to 4.1%. At the same time, patients who receive attenuin and inactivated vaccines are at higher risk of experiencing flu symptoms.
IMPORTANT
Due to the unstable supply of vaccines to the domestic market, in particular, influenza vaccines, there is currently a shortage of the Vaxigrip vaccine.
In this regard, patients who have started a course of vaccination with the Vaxigrip vaccine and are subject to a second vaccination with this vaccine are recommended to continue the course of vaccination with other vaccines that contain strains similar to the Vaxigrip vaccine.
Pediatricians at the Medical Center recommend vaccination (primary, repeated) with the Ultrix and Grippol Plus vaccines.
Please note that every year vaccine manufacturers include in all vaccines the strains of viruses expected in the current season.