Vaxigrip - flu shot
Inactivated (split) flu vaccine
Manufacturer: Sanofi-Pasteur, France.
Protects against viral diseases: influenza types A and B.
Used: for the prevention of influenza in adults and children from 6 months.
Not included in the national vaccination calendar.
Vaccination with the Vaxigrip vaccine is not carried out in clinics
Material and methods
Brief characteristics of the Agrippal S1 vaccine used for immunization of certain groups of the Moscow population during the 2008–2009 epidemic season.
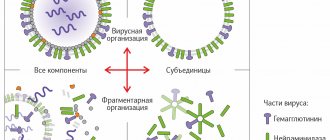
Agrippal S1 is a highly purified inactivated trivalent subunit vaccine for the prevention of influenza of the third generation (Novartis Vaccines and Diagnostics S.r.l., Italy; registration certificate: P No. 012054/01 dated March 23, 2007). The vaccine is registered by the Ministry of Health and Social Development of the Russian Federation and has been used in Russia since 2000. The vaccine is purified surface antigens of influenza viruses type A and B grown on chicken embryo culture inactivated by formaldehyde.
The vaccine complies with WHO recommendations for the 2008–2009 season. The vaccine Agrippal S1 is a colorless transparent liquid. One dose of the vaccine (0.5 ml) contains the following strains of influenza virus: • A/Brisbane/59/2007 (II1N1) similar (A/Brisbane/59/2007, IVR-148) 15 mcg hemagglutinin; • A/Brisbane/10/2007 (H3N2) similar (A/Uruguay/716/2007, NYMC X-175C) 15 μg of hemagglutinin; • B/Florida/4/2006 similar (B/Plorida/4/2006) 15 µg hemagglutinin. Other ingredients: sodium chloride, potassium chloride, potassium phosphate dihydrate, sodium phosphate dihydrate, magnesium chloride, calcium chloride, water for injection. Agrippal S1 does not contain preservatives or mercury compounds, the drug is intended for the prevention of influenza in adults and children from 6 months of age . The vaccine is administered intramuscularly, mainly into the deltoid muscle, or deep subcutaneously, in young children - into the anterior lateral part of the thigh.
The vaccine is produced in a sterile glass syringe of hydrolytic class type I (Eur. Pharm.), 0.5 ml per bottle (1 blister with instructions in Russian).
Contingents of persons participating in the study
This study to evaluate the preventive effectiveness of the Agrippal S1 vaccine was carried out in Moscow. The study involved children (3–6 years old) attending preschool institutions (preschool institutions) and schools (from 1st to 11th grade) of 5 administrative districts of the city (South, South-East, South-West, West), and also medical workers of medical and preventive institutions (HCI) of the Southern Administrative District of Moscow.
Using a random sampling method, experimental and control groups that were equal in all respects were formed among the following contingents: 1. Schoolchildren in grades 1–11: • the main group (O1) vaccinated with the Agrippal S1 vaccine, numbering 5235 people in 5 administrative districts of the city (10 schools in each district) ; • control group (K1) of 4998 people not vaccinated against influenza in 10 administrative districts of the city (10 schools in each district). 2. Children 3–6 years old attending kindergartens: • main group (O2) vaccinated against influenza with the Agrippal S1 vaccine, numbering 4867 people in 5 administrative districts of the city (10 kindergartens in each district); • control group (K2) of 4,901 people not vaccinated against influenza in 10 administrative districts of the city (10 children's educational institutions in each district). 3. Medical workers: • the main group (O3) vaccinated against influenza with the Agrippal S1 vaccine, numbering 7,000 people in 21 health care facilities of the Southern Administrative District; • a control group (CG) of 6,855 people not vaccinated against influenza in 231 health care facilities in 3 administrative districts. Criteria for inclusion in the study: • all children must attend kindergarten or school; • commitment of those participating in the observation. There are no exclusion criteria from the study.
Special conditions
If the first symptoms of ARVI or influenza appeared in persons participating in the study, they were isolated from the team and were placed under medical observation. In each institution where the epidemiological study was conducted, strict records were kept of all patients with influenza and ARVI, both vaccinated against influenza and those not vaccinated.
Method of administration and dosage of the vaccine
To persons from the main groups, the vaccine was administered intramuscularly into the deltoid muscle, 0.5 ml in October 2008. Before vaccination, each child and adult subject to vaccination was examined by a doctor; their body temperature was measured. Persons in the control groups were not vaccinated against influenza during the 2008–2009 epidemic season. due to refusals of vaccinations, however, in the groups that these persons visited, there were children and adults (medical workers) vaccinated against influenza, including vaccines from other manufacturers.
Basic methodological principles for organizing research
The study included schools and kindergartens that experienced the same incidence of influenza and ARVI in the previous epidemic season.
The recording of the incidence of influenza and ARVI began a month after immunization (from December 2008) and was carried out until April 2009 inclusive. All quantitative indicators were processed by the methods of variation statistics.
The epidemiological (preventive) effectiveness of vaccination with Agrippal SI was assessed by comparing the incidence of influenza and ARVI in the main and control groups. The index and efficiency coefficient were determined using the following formulas (Bessmertny B.S., Kheifets L.B., 1963): KE = b/a IE = 100(b–a)/b(%), where: KE – efficiency index, IE is the efficiency coefficient, a is the morbidity rate among vaccinated people, b is the morbidity rate among unvaccinated people.
Assessment of vaccine safety and tolerability
The safety and tolerability of the Agrippal S1 vaccine in the main groups were assessed by the subjective feelings of vaccinated people (complaints) and by data on seeking medical help in the post-vaccination period.
Indications for vaccination "Vaxigrip"
Vaccination is recommended for all individuals and, above all, for those who are at increased risk of contracting influenza, especially if influenza is added to existing diseases:
- Young children (under 1 year old)
- Children attending kindergarten, school, sports and children's sections, clubs.
- Adults over 65 years of age.
- People with chronic respiratory diseases, including bronchial asthma, renal failure, hemoglobinopathies and immunodeficiency conditions.
- Patients with cardiovascular diseases.
- Patients with chronic renal failure.
- Patients with chronic metabolic disorders, including diabetes mellitus.
- Children and adults with immunodeficiency conditions, patients receiving immunosuppressants, cytostatics, radiation therapy or high doses of corticosteroids.
- Children and adolescents from 6 months. under 18 years of age who have been receiving medications containing acetylsalicylic acid for a long time and are therefore at increased risk of developing Reye's syndrome due to influenza infection.
- Medical personnel, teachers, doctors, public transport drivers: all those who come into contact with large numbers of people every day, as well as members of their families.
How is the Vaxigrip vaccine created?
The virus is grown in chicken embryos. When they reach the age of 10–12 days, by double zonal ultracentrifugation, it undergoes thorough purification from proteins and other components and is exposed to detergent.
One dose of the Vaxigrip vaccine in a volume of 0.5 ml contains 15 mcg of hemagglutinin of the virus of each serotype. It is this protein that is responsible for inducing immunity to influenza.
The form of release of the product determines its price. For adults, a dose of 0.5 ml is provided, for children - 0.25 ml.
The Vaxigrip vaccine has the appearance of a slightly opalescent, slightly whitish liquid. The suspension is intended for intramuscular or subcutaneous administration.
Indications for vaccination
Due to the high incidence of influenza and its seasonal nature, it is recommended to vaccinate the population annually in the autumn-winter period.
The vaccine is intended to prevent influenza in adults and children from six months of age. Recommended for people with an increased risk of post-flu complications.
Vaxigrip promotes the formation of specific immunity to current strains of influenza A and B viruses. The development of protection against infections can be discussed 2–3 weeks after vaccination. Immunity remains in humans for 6–12 months.
Contraindications
Only a doctor can decide whether Vaxigrip is suitable for vaccination
Vaxigrip is contraindicated if you have a history of an allergic reaction to any component of the vaccine.
Vaxigrip vaccination is contraindicated in the following cases:
- If a child or adult is allergic (hypersensitivity) to the active substances of the vaccine or any of the ingredients - egg whites or chicken whites, neomycin, formaldehyde or octoxynol-9.
- If the child has an illness with high or moderate fever or acute infection.
Side effects
During formal clinical trials, the most common adverse reactions encountered were:
- increased body temperature;
- decreased performance;
- general malaise;
- feeling of chills;
- headache;
- muscle and joint pain;
- increased sweating;
- hyperemia, swelling, pain at the injection site.
Local adverse reactions pass quickly - within 1-3 days after vaccination. More rare complications include the appearance of transient thrombocytopenia, enlargement of the postauricular lymph nodes, the development of neuritis, vasculitis, encephalomyelitis, and Guillain-Barré syndrome.
Possible side effects
The following adverse reactions may be observed during vaccination: headache, sweating, myalgia, arthralgia, fever, malaise, chills, fatigue, dizziness, insomnia, vomiting, fever. Local reactions may include: redness, swelling, pain, induration, ecchymosis. Reactions usually resolve within 1-2 days and do not require treatment.
Come get vaccinated at MAMARADA. A full range of vaccines for children and adults, family vaccinations - at a special price!
Vaxigrip vaccination scheme
The vaccine is administered intramuscularly or deeply subcutaneously. Children under 9 years of age should receive the dose twice, 4 weeks apart.
Contraindications
The drug is not used in patients:
- with an allergy to chicken eggs, chicken protein and other components of the drug;
- elevated body temperature and other symptoms of an acute infectious disease (vaccination is postponed until complete recovery).
The reactogenicity of the Vaxigrip vaccine is low. In children from three months to nine years (1.9%) during primary vaccination, local reactions are practically absent; general reactions in the form of chills, headache, fever, and malaise are possible. There are no reactions after repeated immunization.
Pregnancy and lactation
The Vaxigrip vaccine can be used during breastfeeding. Pregnant women are allowed to administer the drug from the second trimester (with a high risk of developing post-flu complications, regardless of the period). No negative effects of immunization on the fetus or the body of a pregnant woman were found.
You can get the Vaxigrip vaccine at the Inpromed clinic or by inviting a doctor to the organization. Check the prices for vaccination drugs in the price list on the website.
As it was before
The lack of foreign vaccines during the epidemiological season is an annual problem. In 2021, according to Vedomosti, Vaxigripp produced by Sanofi Pasteur was delivered only by the end of September, while Influvac was not delivered at all. In 2021, according to Rossiyskaya Gazeta, at the beginning of October it was impossible to be vaccinated with a foreign vaccine in Russia.
Sanofi told RBC that in 2021 they plan to continue supplying the vaccine to prevent influenza. “The vaccine will arrive in the country to further undergo the procedure for introducing it into civilian circulation and no earlier than in October, it will be available for shipment from the warehouse of Sanofi Russia JSC. The vaccine will be available through the traditional distribution network,” Yuri Mochalin, director of corporate relations at Sanofi in the Eurasian region, explained to RBC.
Abbott did not respond to RBC’s request about whether it plans to supply the Influvac vaccine to Russia.
Choice without choice
Almost the entire volume of both federal and city supplies in St. Petersburg last season was the domestic Sovigripp vaccine. The drug is produced by , which works within the structure of JSC National Immunobiological Rostec. Also, a subsidiary of Rostec is the Fort enterprise, which produces the drug Ultrix, which occupies 20% of the Russian market.
According to the National Immunobiology Company, the volume of influenza vaccines ordered in the country more than doubled from 2006 to 2017. In the current epidemiological season, 62.3 million doses of Sovigrippa and Ultrix were purchased for centralized supplies to the regions. According to the law, only domestic drugs are purchased for mass vaccination, which is carried out to patients at state expense.
According to the city health committee, in St. Petersburg in the 2018/19 season, the share of imported vaccines as part of universal vaccination was 1.32%. These are 1,760 doses of Vaxigrip vaccines purchased for people who suffer from chronic diseases and cannot take Sovigripp.
Vaxigrip is a product of the French company Sanofi Pasteur. According to the Health Committee, these purchases were carried out by district administrations.
Having been present on the Russian market for about 25 years, the French pharmaceutical manufacturer works mainly through pharmacy chains and distributors. The volume of supplies of the French vaccine to Russia has remained unchanged for several years: the company retains a market share of about 1–2%.
Also in Russia are influenza vaccines from NPO Petrovax Pharm, the Research Institute of Vaccines and Serums and the American company Abbott. In St. Petersburg, the Research Institute of Influenza and the Research Institute of Experimental Medicine are also working on the development of this group of drugs. The main difficulty is not even the cost of development, but the ability to find a suitable production site, because this is a necessary condition for conducting clinical trials.
The Research Institute of Vaccines and Serums brought its drug “Flu-M” to the market in 2018 and, as a result, has already won a 1% market share. Over the 11 months of 2021, the institute produced and sold 24,120 doses of its influenza vaccine for 5.98 million rubles.
As for release forms, vaccines are produced in three modifications - syringes, ampoules and vials. Moreover, if in 2017 the most popular form among consumers were syringes, then in 2018 the primacy passed to ampoules. The average price per dose in the 2018/19 season ranged from 162 rubles (Sovigripp) to 247 rubles (Vaxigrip). According to analytical estimates, the weighted average price per dose of influenza vaccine increased by 5% compared to 2017.