What is Vaxigrip?
This is an inactivated split vaccine: the influenza viruses it contains are killed and split into individual proteins. Cleavage allows the body to better recognize viral proteins and develop protection against each. Vaxigrip is designed to protect against the most current strains of the influenza virus. For the 2019/2020 season these are:
- A/Brisbane/02/20189 (H1N1)pdm09 - similar (A/Brisbane/02/2018, IVR-190)
- A/Kansas/14/2017 (H3N2) - similar (A/Kansas/14/2017, NYMC X-372)
- B/Phuket/3073/2013 - similar (B/Phuket/3073/2013, wild type)
- B/Colorado/6/2017 - similar (B/Maryland/15/2016, NYMC BX-69A)
- Auxiliary components (2009/2010)
Who is suitable for the Vaxigrip vaccine?
All adults and children over 6 months can be vaccinated. When vaccinating children under 9 years of age who have not previously been vaccinated against influenza with this or other vaccines, two doses are required with an interval of 4 weeks.
WHO recommends getting vaccinated against influenza for people at risk:
- preschoolers and schoolchildren;
- people over 65 years old;
- “owners” of chronic diseases of the heart, lungs, kidneys, liver, metabolism, people with chronic neurological or immunodeficiency conditions;
- health care workers, especially those working with the elderly and infants.
Vaxigrip - flu shot
Inactivated (split) flu vaccine
Manufacturer: Sanofi-Pasteur, France.
Protects against viral diseases: influenza types A and B.
Used: for the prevention of influenza in adults and children from 6 months.
Not included in the national vaccination calendar.
Vaccination with the Vaxigrip vaccine is not carried out in clinics
Side effects
- The following reactions to the vaccine are observed with a frequency of 1% to 10%:
- fever, malaise, chills, headache;
- muscle and joint pain;
- local reactions: redness, swelling and pain at the injection site.
These reactions disappear within 1-2 days and do not require special treatment.
You cannot get the flu from the inactivated split vaccine because it does not contain live viruses.
Flu and vaccines against it
Acute respiratory viral infections ( ARVI ) are a group of diseases that are caused by many pathogens (influenza viruses, parainfluenza, respiratory syncytial virus, adenovirus, metapneumovirus, etc.), transmitted by airborne droplets and characterized by acute damage to the human respiratory system. The most common symptoms of ARVI: redness of the mucous membranes of the oropharynx, sore or sore throat, especially when swallowing, runny nose or nasal congestion, hoarseness, coughing, sneezing, fever, often to small values (not higher than 38 ° C).
Influenza is an acute respiratory infection caused by the influenza virus, characterized by high fever (38-40 ° C), severe general intoxication and damage to the respiratory tract, often in the form of tracheitis.
The incubation period (i.e., the time from the moment of infection to the appearance of the first signs of the disease) for seasonal influenza ranges from 12 to 48 hours, with the highly pathogenic version extending to 5-7 days. Influenza is characterized by an acute onset with chills, an increase in temperature to maximum values already on the first day of illness and general symptoms of intoxication (severe weakness, fatigue, headache in the frontal areas, aches in the muscles, bones, joints, pain in the eyeballs, photophobia, lacrimation etc.). After a few hours, the above manifestations of the disease are accompanied by signs of damage to the respiratory tract, most often in the form of nasal congestion or mild runny nose, sore throat, painful dry cough, raw pain behind the sternum and along the trachea, and hoarse voice. For most flu patients, complete recovery occurs within 7-10 days. However, there is a group of people prone to complications from this disease, and they constitute a risk group for severe acute respiratory viral infections and influenza. First of all, these are children under 1 year of age, all persons over 65 years of age, pregnant women, patients with chronic diseases of the cardiovascular and respiratory systems, with immunodeficiency conditions, with inadequately controlled diabetes mellitus and other severe concomitant pathologies. This category of people should immediately contact specialists when the first symptoms of influenza appear, because only early initiation of therapy can prevent the development of severe complications (pneumonia, exacerbations of chronic obstructive pulmonary disease, sinusitis, otitis, etc.).
Most patients with influenza who do not belong to risk groups with mild to moderate uncomplicated course of the disease can be treated on an outpatient basis.
Maintain semi-bed rest, good nutrition and drink plenty of fluids (up to 1.5-2.0 liters of fluid per day). Drinking plenty of fluids helps eliminate microbial waste products and reduce intoxication. In addition, increased drinking regimen helps to cope with fluid loss as a result of heavy sweating during fever and helps to thin sputum and help clear it when coughing.
Taking paracetamol and other antipyretics (ibuprofen, ibuclin, nimesulide, combined antipyretic drugs Theraflu, Coldrex) in standard dosages. As a rule, in previously healthy adults and children, antipyretics are prescribed at a temperature above 38.5 ° C; lowering the temperature is not recommended. For children under 18 years of age, the use of aspirin is contraindicated against the background of any acute respiratory viral infection!
It is necessary to carry out symptomatic treatment of rhinitis, pharyngitis, tracheitis (use vasoconstrictor or herbal nasal drops, antitussives and sputum thinners, rinsing the throat with decoctions of chamomile, sage, calendula, solutions of furacillin, orasept, etc.).
Antiviral therapy is prescribed only by a doctor in an individual dose depending on the clinical picture and severity of the disease, as well as the presence of concomitant diseases. The most effective use of antiviral drugs is in the first 48-72 hours from the onset of the disease.
It should be remembered that unjustified independent use of antibiotics without appropriate indications not only does not prevent the development of bacterial complications, but can also worsen the course of the underlying disease due to the development of side effects of the drug (especially allergic reactions, dysbacteriosis), and also contribute to the formation of resistant forms of bacteria. .
If there are patients with ARVI and influenza in the family, you need to remember the principles of preventing the transmission of infection to family and friends (isolating the sick person in a separate room, wearing masks at home, observing personal hygiene rules, regularly airing rooms and wet cleaning, limiting visits of other people to the patient).
First of all, all people belonging to the risk group, pregnant women, people suspected of developing pneumonia, people with severe influenza, patients with a risk of developing life-threatening conditions are hospitalized.
Prevention methods:
Avoid close contact with people who seem unwell, have fever and cough
- During the epidemic, avoid crowded places or reduce the time you spend in them
- try not to touch your mouth and nose
- Practice good hand hygiene - wash your hands frequently with soap and water or use an alcohol-based hand sanitizer, especially if you touch your mouth or nose.
- increase the flow of fresh air into living spaces, open windows as often as possible.
- Use protective masks when in contact with a sick person.
- adhere to a healthy lifestyle: adequate sleep, balanced nutrition, physical activity, regular walks in the fresh air.
If you do get sick, then you need to:
- If you feel unwell, stay at home and follow the doctor’s recommendations, if possible keep a distance from healthy people
- rest, take plenty of fluids
- Cover your mouth and nose when coughing or sneezing with a handkerchief or disposable tissues. After use, the scarf or napkin must be disposed of immediately or washed and ironed
- wear a mask if you are in a common area of the house near other people
- Tell your family and friends about the illness.
Wearing masks is mandatory for people who have close contact with a sick patient. Used masks should be replaced with new ones as soon as the previous mask becomes damp (damp), preferably at least once every 3 hours.
One of the most effective methods of specific prevention is vaccination. According to WHO recommendations: “Vaccination is the basis for the prevention of influenza,” since it reduces the spread of the disease and mortality, is 3-4 times cheaper than the cost of treating influenza and its complications, allows you to save money on the treatment of influenza and its complications, and can significantly reduce temporary disability, available, effective and safe for adults, children from 6 months of age, pregnant women. Currently in the Republic of Belarus there are several types of influenza vaccines (live intranasal, Grippol, Vaxigrip, Influvac). First of all, patients at risk for severe influenza, women in the 2nd and 3rd trimesters of pregnancy, children, medical workers and social service workers should be vaccinated. The optimal time for vaccination for countries in the Northern Hemisphere is from October to mid-November. Immunity develops within 2 weeks after the vaccine is administered. You should remember the well-known principle that it is better to prevent a disease than to treat it.
Another option for specific prevention of influenza is the use of drugs with antiviral activity (oxolinic ointment, arpetol, oseltamavir, etc.). In order to reduce the likelihood of infection with influenza, 0.25% oxolin ointment is applied to the nasal mucosa twice a day (morning and evening). Arpetol is used for prophylaxis in children 2-6 years old, 50 mg/day orally, 6-12 years old, 100 mg/day, over 12 years old, and in adults, 200 mg/day for 14 days. Oseltamavir (Tamiflu) is prescribed for prophylaxis in children over 12 years of age and adults, 75 mg orally once a day for 5 days. Indications for drug prevention of influenza are: having a family member with influenza, contact with someone with influenza, people at high risk for severe influenza, medical personnel (if they are not vaccinated).
Remember, self-medication for the flu is unacceptable, especially for children and the elderly. After all, it is impossible to predict the course of the flu, and complications can be very different. Patients with influenza require constant monitoring by health workers, and late hospitalization leads to a protracted course of pneumonia and other complications and an increased incidence of deaths.
Only a doctor can correctly assess the patient’s condition, prescribe adequate treatment with antiviral agents and antibacterial drugs, and conduct additional examination methods for the patient (clinical tests, chest x-ray, ECG, etc.).
Your health is in your hands, and early contact with a specialist is the key to success in maintaining it.
Types of Flu Vaccines
For specific prevention against influenza, vaccines are used, divided into two large groups:
- live vaccines (containing weakened and non-infectious viruses);
- inactivated (does not contain live viruses).
Inactivated vaccines are divided into three groups:
- whole cell - contain whole cells of the influenza virus;
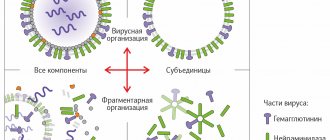
- split or split vaccines - contain split influenza virus cells containing both surface and internal protein molecules and antigens, due to the high degree of purification they do not contain viral lipids and proteins of the chicken embryo (Vaxigrip vaccine (France));
- subunit vaccines - contain only surface viral proteins (hemagglutinin and neuraminidase) (Grippol plus vaccine (Russia), Influvac vaccine (former Solway Pharma, Netherlands)).
The following influenza vaccines are currently used for vaccination in Minsk:
- Grippol plus (Russia) – for free and paid vaccination;
- Influvac (Netherlands) – for paid vaccination;
- Vaxigrip (France) – for paid vaccination.
Types of modern influenza vaccines
1. Whole virion and live vaccines (first generation vaccines)
The development of influenza vaccines began in the early 40s under the auspices and with the support of the US military department, since the massive incidence of influenza epidemics was regarded as a threat to the combat effectiveness of troops. The very first vaccines contained whole viral particles (live attenuated and killed inactivated). The vaccines were produced by inactivating the virus (grown in chicken embryos) with formaldehyde. The effectiveness of such vaccines was quite high. However, due to insufficient purification, these vaccines contained a large amount of chicken protein, and therefore were highly reactogenic: vaccination was often accompanied by high fever, induration and pain at the injection site .
For half a century, vaccines have been continually improved, their potency and purity continually improved. In 1966, a new technological purification method (zonal centrifugation) was used, which made it possible to achieve better purification of the vaccine from egg whites and various cell components (which were the cause of the most frequent and serious reactions to the vaccine). These highly purified vaccines were less reactogenic than the earliest vaccines, but still, especially in children, often caused systemic reactions (headache, fever and malaise). This was due to the high content of viral components in the vaccine, which stimulated the production of interferon to the same extent as occurs during infection with a wild virus. The increased concentration of interferon in the body is the cause of “side effects”, which are considered as general reactions to the administration of the vaccine.
Today, the inactivated whole virion vaccine contains whole influenza viruses that have undergone preliminary inactivation and purification. When making a vaccine, the influenza virus is grown in chicken embryos, then isolated and inactivated using modern methods. These vaccines have good immune response rates, but they are highly reactogenic and therefore cannot be used in children.
2. Split vaccines (second generation)
The use of diethyl ethers as solvents made it possible to break the lipid membrane of the virus into parts, which was the basis for the appearance of split vaccines in the 60s.
Split vaccines contain particles of the destroyed virus - surface and internal proteins. The vaccine is made by breaking down viral particles using organic solvents or detergents. Split vaccines are characterized by a significantly lower risk of adverse reactions, presumably due to the destruction of the spatial structure of the virus.
The following split vaccines are registered and approved for use in Russia: begrivak (Germany); Vaxigrip (France); fluarix (Belgium).
3. Subunit vaccines (third generation)
In the 70s, it was proven that the most significant antibodies for providing protection against influenza are specific antibodies against the surface antigens of viruses (hemagglutinin and neuraminidase), and not against the internal proteins of the virus (nucleoprotein). Thus, a vaccine containing only surface antigens and devoid of viral components was expected to have reduced reactogenicity with equal effectiveness.
The first subunit vaccine appeared in 1980 . It contains only two surface glycoproteins - hemagglutinin and neuraminidase and is maximally purified from protein . With proven identical immunogenicity as whole-virion and split-virion vaccines, subunit vaccines are less reactogenic, as evidenced by the results of the analysis.
Due to its high efficiency and low reactogenicity, this vaccine can be used in children from 6 months of age.
The composition of vaccines changes every year in order to provide maximum protection against the “wild” virus. Depending on WHO forecasts (what type of virus will circulate next season), different viral antigens are included in the vaccine. The vaccines include 3 types of viral antigens - 2 types A and 1 type B.
The following subunit vaccines are registered and approved for use in Russia: Agrippal (Germany); influenza (Russia); influvac (Netherlands)