The choice of vaccination for a particular patient is the competence of the doctor.
Photo: Vladimir VELENGURIN
Today in our country there are already four Russian coronavirus vaccines used for mass vaccination (see below). On June 26, it became known that the first batches of the Sputnik Light vaccine were released into civilian circulation (that is, for the same general vaccination). And by the end of June, according to Health Minister Mikhail Murashko, about 2.5 million doses of this vaccine will arrive at vaccination centers.
As the Ministry of Health and Rospotrebnadzor explain, the choice of vaccination for a particular patient is the competence of the doctor. In this case, the doctor needs to take into account the composition of each vaccine, contraindications, age and health status of the patient. As of today, there are no official recommendations recorded in documents about which categories of citizens are best suited for this or that vaccine.
At the same time, in practice, we see that doctors at vaccination points are often overloaded, and the flow of people wanting to get vaccinated is growing. And it is not always possible for doctors to interview each person who comes in detail, to delve deeply into the features of his condition, the composition of a particular vaccine, and the list of contraindications. Therefore, we have compiled a detailed analysis of the differences between vaccines - it will help you navigate, including asking the right questions to the doctor before vaccination. We also present the opinions of leading experts on vaccinations, medications and infectious disease doctors - what is important to pay attention to when choosing vaccinations.
HOW THE VACCINE WORKS
“Sputnik V”: A piece of the coronavirus genome is added to a neutralized adenovirus (a type of cold virus) and delivered to human cells. In response to the appearance of a foreign fragment, the body triggers the production of immunity. An important feature: for the vaccine to work better, two different delivery adenoviruses are used: ad-26 for the first injection and ad-5 for the second injection.
EpiVacCorona: Contains artificially created small fragments of coronavirus proteins (peptides). They are connected to a carrier protein and attached to an auxiliary substance (adjuvant) - aluminum hydroxide. Once in the body, they trigger the production of an immune response.
CoviVac: The vaccine contains whole viral particles that are inactivated - that is, dead and cannot cause any harm to the human body. But they can cause the development of immunity against covid.
Sputnik Light: This is the first component of the Sputnik V vaccine - it works the same way. The difference is that Light will provide shorter-term immunity to previously unvaccinated people than Sputnik V. When vaccinating those who have recovered from or have previously been vaccinated (revaccination), it is expected to provide an optimal level of protection.
The effectiveness of Russian vaccines against coronavirus
Most of the data has been published on the Sputnik V vaccine. According to the results of all clinical trials, the drug demonstrated an effectiveness of 91.6%. The effectiveness rate for older people is practically the same - 91.8%.
The effectiveness of EpiVacCorona and CoviVac will become known after the completion of the third phase of trials. The developers claim that immunity is formed in 100% of participants.
Meanwhile, participants in the EpiVacCorona trials express doubts about the effectiveness of the drug: in an open letter to the Ministry of Health, the initiative group states that antibodies do not form in a third of the volunteers vaccinated with the vaccine. Among those vaccinated, there are also cases of severe coronavirus. When conducting their own study of the vaccine on the formation of neutralizing antibodies in the body, the authors of the letter, including molecular biologist of the Ministry of Health Denis Lagutkin, found that not a single sample of serum with antibodies could neutralize the virus in the laboratory.
It remains to wait for publications with the results of vaccine trials; perhaps they will be able to prove the effectiveness of the drug.
WHEN IMMUNITY APPEARS
“Sputnik V”: 2-3 weeks after the first injection, partial immune protection, 21 days after the second injection - full immunity to covid.
EpiVacCorona: On average 32-42 days after the first vaccination.
CoviVac: On average 42 days after the first vaccination.
“Sputnik Light”: 28 days after the injection during primary vaccination; on the 10th day after the injection in those who have recovered and previously vaccinated.
According to published data, the Sputnik V vaccine has a protective ability against the Indian strain of coronavirus and a stronger development of immunity.
Photo: Vladimir VELENGURIN
About the service
Vaccination in MEDSI clinics is carried out under the supervision of a qualified specialist in accordance with the National Vaccination Calendar. In addition, vaccination is carried out against some infections that are not included in the list of mandatory vaccinations, including hepatitis A, human papillomavirus, tick-borne encephalitis, rotavirus infection, meningococcal infection, and chickenpox.
The clinics use only high-quality domestic and imported vaccines. MEDSI specialists closely monitor trends in the pharmaceutical market and promptly purchase new generation vaccines for the clinics’ arsenal, that is, more effective and safe ones.
Vaccination is carried out in MEDSI clinics by appointment at a time convenient for you, subject to availability of the vaccine and after examination by a doctor. You can make an appointment and find out more information about the availability of the vaccine you need at MEDSI clinics by calling the 24-hour contact center +7 (495) 7-800-500.
Annual flu vaccination – protecting adults and children
Influenza is an acute infection that causes annual epidemics. It occurs with high fever for 3-5 days, with severe intoxication in the form of headaches and muscle pain. Influenza exacerbates chronic inflammatory processes in the body, thereby causing high mortality from complications.
The causative agents are pneumotropic RNA-containing viruses of 3 serotypes (A, B, C).
The spread of influenza in modern times is largely due to the rapid movement of populations.
Vaccination is the most effective method of reducing the incidence of not only influenza, but also acute respiratory infections caused by other respiratory viruses, both among adults and among children, including patients with bronchial asthma and respiratory allergies.
Immunity is developed 14 days after vaccination.
The constant mutation of influenza virus strains, as well as the short duration of the post-vaccination period (6-12 months), require annual repetition of vaccinations, even if its strain composition has not changed compared to the previous season. When infected with strains of influenza virus different from the vaccine ones, the disease in vaccinated people is milder. Live vaccines are weakly reactogenic; temperatures above 37.5 in the first 3 days are allowed in no more than 2% of vaccinated people. Subunit vaccines produce weak short-term (48-72 hours) reactions in no more than 3%. According to international independent studies, subunit vaccines are the least reactogenic.
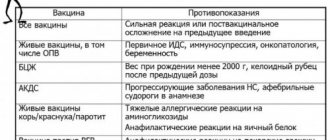
Contraindications for all vaccines are allergies to chicken egg whites, allergic reactions to the administration of any influenza vaccine. All influenza vaccines are prepared from the current strains of viruses A/H1N1, A/H3N2, B, recommended annually by WHO.
HOW LONG DOES IMMUNE PROTECTION LAST?
"Sputnik V": According to the instructions, "the duration of protection is unknown." According to the developers, from 1 to 2 years, but during a surge in incidence, revaccination is recommended every 6 months.
EpiVacCorona: According to the instructions, “the duration of protection is unknown.” According to the developers - presumably up to one year.
CoviVac: According to the instructions, “the duration of protection is unknown.” According to the developers - presumably up to one year.
“Sputnik Light”: According to the instructions, “the duration of protection is unknown.” According to the developers, on average 4-5 months.
WHAT STAGE OF RESEARCH IS THE VACCINE AT?
“Sputnik V”: The results of the phase I-II clinical trial have been summed up - this means that safety and immunogenicity have been tested, that is, the ability to ensure the production of antibodies. Preliminary interim results of a post-registration phase III clinical trial have also been obtained, during which the effectiveness of protection against covid is tested in real life. The study completion date is December 31, 2022. The stated number of volunteers is 40,000 people.
EpiVacCorona: Interim results of the phase I-II clinical trial have been summed up. Its completion date is December 30, 2021. In parallel, a post-registration clinical trial of phase III-IV is ongoing, the completion date is 08/31/2021. The declared number of volunteers is 4,991 people.
"CoviVac": Interim results of the phase I-II clinical trial have been summed up. Its completion date is December 31, 2021. In parallel, a post-registration phase III clinical trial is ongoing, with a completion date of 12/30/2022. The stated number of volunteers is 32,000 people.
“Sputnik Light”: Interim results of the phase I-II clinical trial have been summed up. Its completion date is December 31, 2021. In parallel, a post-registration clinical trial of phase III-IV is ongoing, the completion date is 01/28/2022. The stated number of volunteers is 7,000 people.
How the vaccines were tested
All three vaccines were registered before the end of clinical trials. In normal times, this would be a violation of the drug registration procedure, but during the pandemic, the scheme for releasing vaccines into civilian circulation was relaxed. If this had not been done, coronavirus vaccines would not have appeared until several years later.
Before testing vaccines on humans, the drugs were tested in experiments on laboratory animals, including primates.
Tests of Sputnik V or Gum-COVID-Vaka.
By August 2021, the first two phases of clinical trials of the vaccine were completed. 76 volunteers aged from 18 to 60 years took part in them. The study was open-label and non-randomized, meaning no placebo was used and all participants received the vaccine. It was concluded that the drug was well tolerated and produced an immune response. None of the volunteers became infected with coronavirus after the vaccine.
The third phase of testing began after the registration of Sputnik V. It included more than 30,000 participants who were given either the vaccine or a placebo. The drug was also tested on volunteers aged 60 to 84 years. The trial results were published in February 2021 in the Lancet.
EpiVacCorona trials
The first two phases of clinical trials of the vaccine were completed by the end of September 2021. 14 volunteers took part in the first phase, 86 - in the second. All of them were aged from 18 to 60 years. The first phase was carried out in the same way as Sputnik V: the volunteers knew which drug they were receiving. The second phase was already carried out using a placebo, the participants did not know which drug they were being given.
The third phase of testing is now ending. It started in November last year. The vaccine developers plan to publish the test results in a scientific publication in the near future, although they have not yet said which one. Nothing is known yet about preliminary test results.
In February, it became known that Vector had completed testing the drug on older people. The vaccine has shown high safety and immunogenicity.
Trials of “CoviVac” or “Chumakov vaccine”
During the first two phases of testing, about 200 volunteers aged 18 to 60 received the vaccine. They ended at the end of last year. According to the developers, the drug is safe for people and causes an immune response.
A phase three trial involving 3,000 people is planned for April. Children and elderly people will also take part in it.
VACCINE EFFECTIVENESS
“Sputnik V”: More than 91% according to the interim results of the phase III clinical trial.
EpiVacCorona: Unknown. According to the developers, during phase I-II clinical trials, antibodies were produced and retained in 100% of volunteers 6 months after vaccination (note that the production of antibodies does not provide a 100% guarantee of equivalent vaccine effectiveness in practice).
CoviVac: Not known. According to the developers, during phase I-II clinical trials, antibodies were detected in 85% of volunteers (the production of antibodies does not provide a 100% guarantee of equivalent vaccine effectiveness in practice).
Sputnik Light: Unknown. According to the developers - about 70%.
STORAGE CONDITIONS
"Sputnik V": Frozen drug - at a temperature not exceeding minus 18 ° C. Liquid preparation - at a temperature of 2 to 8 °C for no more than two months.
"EpiVacCorona": At a temperature of 2 to 8 ° C. Cannot be frozen.
"CoviVac": At a temperature of 2 to 8 ° C. Cannot be frozen.
"Sputnik Light": Frozen drug - at a temperature not exceeding minus 18 °C. Liquid preparation - at a temperature of 2 to 8 °C for no more than one month.
WHAT FOREIGN COUNTRIES CAN YOU ENTER WITHOUT AN ADDITIONAL PCR TEST?
Sputnik V: Cyprus, Turkey, Maldives, Croatia, Montenegro, Bulgaria, Armenia, Georgia; Jordan (a PCR test is not required upon arrival, but must be done 72 hours before departure from the Russian Federation).
EpiVacCorona: Maldives
CoviVac: Maldives
"Sputnik Light": Maldives
Update as of June 30, 2021: any Russian vaccine to enter without PCR tests . This was announced on June 30 by the head of the Turkish Ministry of Foreign Affairs, Mevlut Cavusoglu.
WHAT ARE THE ADVANTAGES OF THE VACCINE
Sputnik V: vaccination provides the highest level of antibody production. It is assumed that due to this, the vaccine provides the most powerful and longer-lasting protection.
"EpiVacCorona": considered the "lightest". As the developers explain, the immune system is not burdened with the production of antibodies, which play a lesser role in the fight against the disease. And due to this, vaccination is easier to tolerate; there are practically no post-vaccination reactions.
“CoviVac”: vaccination imitates the natural process of encountering an infection, only neutralized. Due to this, there are almost no side effects during vaccination and at the same time, a wide range of antibodies is formed to different fragments of the coronavirus.
“Sputnik Light”: allows you to quickly, after just one injection, receive immune protection against covid (albeit relatively short-term).
COVID-19 vaccines: choose the best one and stay alive
According to the VTsIOM study “The First Year of COVID‑ 19 in the Mirror of Public Opinion,” 57% of Russians are in favor of mass vaccination. Globally, the share of supporters of the vaccination campaign is even greater: in the USA - 66%, in Germany - 67%, in the UK - 75%. The leaders in the number of vaccinated people are the USA, China, the European Union, Great Britain, and Israel, and the number of people who have received the vaccine has already exceeded 100 million people. And if there is relative unanimity on the issue of the need for universal vaccination to defeat the coronavirus, then debates about the quality of vaccines do not subside - which is more effective and safe? Let's try to figure it out.
Artist: Yuri Aratovsky
Classic or avant-garde?
It usually takes several years to create a new vaccine, but in the emergency of a pandemic, things have moved much faster. In a variety of countries, dozens of vaccines—candidates for saving humanity—were developed in record time. According to WHO, more than 169 vaccine candidates against COVID‑19 are currently under development, of which 26 vaccines are already undergoing clinical trials in humans.
There are different classification systems for vaccines. The simplest one suggests conditionally dividing vaccines into two groups: classical (recombinant, peptide, virus-inactivated) and genetic (vector, DNA, mRNA). The first deliver ready-made antigens into the body, which are viral proteins, their fragments (peptides) or inactivated viral particles. The latter produce a response at the gene level and contain not the virus itself or its protein, but the genetic material of SARS‑COV‑2.
Rospotrebnadzor divides vaccines against the new coronavirus infection into five types.
- Subunits are derived from antigenic components, such as proteins, and usually have low reactogenicity (few side effects), but require booster vaccinations to enhance the immune response.
- Virus-like particle vaccines mimic the structure of the virus but do not contain its genetic material. They are safe, effective, but have many technological difficulties in production.
- Whole-virion ones are created using classical technology, close to the natural mechanism of immunity formation, and contain a weakened or inactivated virus.
- Vectors deliver the genetic material of the virus into the cell using a vector - another virus that is not able to reproduce in the human body. They are highly immunogenic, but the formation of an immune response to the vector virus does not guarantee an adequate immune response to the target virus.
- Nucleic acid-based vaccines are genetically engineered constructs based on DNA and RNA that ensure the synthesis of the desired viral protein, after which the formation of an immune response occurs. These vaccines are relatively new, so experts cite the possibility of unexpected side effects in the future among the disadvantages.
It turns out that whole-virion vaccines are a classic. They have been well studied and have the longest history of use, but their production is long and labor-intensive and requires compliance with special sanitary standards and biological safety control. DNA and mRNA (messenger RNA) vaccines are the vanguard. Their production is one of the simplest, but we still don’t know enough about the technologies and consequences of delivering genetic material inside the cell.
"Dude" and company
Now Russians are actively being vaccinated with the two-component vaccine Gam‑COVID‑Vac, better known by its name. Vaccination with EpiVacCorona has started, but is still in the final stage of clinical trials. A vaccine from the Federal Scientific Center for Research and Development named after M.P. Chumakov of the Russian Academy of Sciences (comic working title “ChuVak”) is approaching.
How do they differ from each other, as well as from the most famous foreign vaccines developed by Pfizer, Moderna, Astra Zeneca?
- Gam-COVID-Vac (“Sputnik V”) was developed by NICEM named after. N. F. Gamaleya. This is the world's first registered vector vaccine based on a new technology platform - two human adenoviruses carrying the coronavirus protein gene. The vaccine should be stored at a temperature of -18 °C. During final clinical trials, Sputnik V showed an effectiveness of 91.4%.
- “ EpiVacCorona ” is not a vector vaccine, but was invented by the Federal Budgetary Institution State Scientific Center for VB “Vector”, which is why confusion often occurs. In fact, this vaccine is subunit, containing synthetic peptides identical to the virus’s own proteins. The developers used three types of antigen to enhance immunogenicity, as well as carrier proteins and excipients that are needed for the penetration of vaccine components into the body. Russians are well familiar with such vaccines; they are vaccinated against influenza with the subunit domestic vaccine “Grippol Plus”. The same type includes vaccines against pneumococcal and meningococcal infections. Referring to the results of the first and second phases of clinical trials, at the end of January Rospotrebnadzor announced one hundred percent immunological effectiveness of the vaccine.
Vaccine Institute named after. M.P. Chumakov RAS so far has only working versions of the names (“ChuVak”, “CoviVak”), it is not registered, but it has already been adopted by the former chief sanitary doctor of the Russian Federation, academician of the Russian Academy of Sciences Gennady Onishchenko . According to him, vaccination with this whole-virion vaccine “proceeded adequately.” This class of vaccines includes, for example, vaccinations against whooping cough, tick-borne encephalitis, and hepatitis A. According to preliminary results of clinical trials, the immunological effectiveness of the drug is 70% two weeks after the first vaccination, 90% three weeks after revaccination. According to Academician of the Russian Academy of Sciences, chief researcher at the Chumakov Center, Professor Alexei Egorov , it is the content of the whole virus, and not its individual fragments, that ensures the formation of a complete set of antibodies - the vaccine, its creators promise, will be equally effective against all strains of coronavirus.
- BNT162b2 ( Pfizer) , a messenger RNA vaccine created jointly by the German biotechnology company BioNTech and the American Pfizer, was the first to be registered in the European Union. According to clinical trials, the vaccine demonstrates 95% effectiveness, which requires two doses administered three weeks apart. Doctors say the main disadvantage of the vaccine is the strict storage conditions - at a temperature not exceeding -70 °C.
- mRNA-1273 ( Moderna ) is based, like the Pfizer , on messenger RNA encoding the coronavirus S protein gene. The effectiveness of the vaccine developed by the American company Moderna, according to clinical trials, was 94.5%. The vaccine can be stored in a regular refrigerator at temperatures up to 8 °C.
- AZD1222 (Astra Zeneca) by the British-Swedish company Astra Zeneca is a vector vaccine. It is made on the basis of an adenovirus, but, unlike the Russian Sputnik V, it used a chimpanzee adenovirus, not a human one. The effectiveness of the vaccine, according to the results of clinical trials, was 70%. But after some time it turned out that it can be increased to 90% if the dosage is chosen correctly (during primary vaccination, a small dose of the drug is administered, and with secondary vaccination, the dose is increased).
Risks of vaccination
Efficiency is one “leg” of vaccination. The second is safety. If the effectiveness or safety is not up to par, vaccination is lame and doubts arise about its necessity. Is it worth rushing and offering your forearm to the doctor to the cheerful lines “I’m not afraid of vaccinations, if necessary, I’ll inject myself” (it seems that they have become the refrain of the current vaccination campaign)?
Yes, more than half of the Russians surveyed are in favor of mass vaccination. But there are other numbers. Almost 30% of compatriots doubt whether to go to vaccination clinics. After all, complications are possible. We are afraid - and our fear is completely understandable. Before the COVID‑19 pandemic, no vaccine against an infectious disease had been developed in less than a few years. Before the pandemic, there was no vaccine against human coronavirus infection. Before the pandemic, no RNA vaccine had been licensed for use in humans... The unknown is scary. And there are many unknowns in the equation called coronavirus vaccination.
In mid-January, WHO even convened a forum dedicated to identifying gaps in knowledge about vaccines against SARS‑CoV‑2. About 3,000 scientists from 130 countries participated in the forum; it was decided to organize events to exchange knowledge and experience regularly. So far, information about the safety of anti-Covid vaccinations is contradictory. The Ministry of Health warns of side effects similar to the symptoms of ARVI: fever, weakness, drowsiness, chills. There are reports in the media about new complications after vaccination: paresis of the facial nerves, severe allergic reactions...
According to the head of the Department of Epidemiology and Evidence-Based Medicine at Sechenov University, chief freelance epidemiologist of the Russian Ministry of Health, Academician of the Russian Academy of Sciences Nikolai Briko , although there is still too little reliable data regarding the new coronavirus, the entire history of mankind’s fight against viruses shows: there is no surer way to defeat an infectious disease than vaccine prevention.
“The only way to stop the spread of infection is to create herd immunity. Before the start of vaccination, it was created at the expense of those who had recovered from the disease. However, with this method of creating herd immunity, the cost is too high - excess mortality,” Bricot said.
Nikolai Briko believes that coronavirus will become one of the seasonal diseases and will join the army of etiological agents of ARVI, among which there were already four coronaviruses. The situation must be met head on, strengthening the immune system with a healthy lifestyle and vaccinations. Which one is better? Doctors answer the question evasively - too little data has been accumulated.
“All three Russian vaccines against the new coronavirus infection were created on different technological platforms, and today it is very difficult to assess which one is better. There are neither exact evaluation criteria nor sufficient experience and knowledge. It is clear that those who have recovered from COVID‑19 are protected from infection for an average of 6–8 months, and as long as antibodies remain, there is no need to be vaccinated. It is clear that only vaccines created on the same platform can be combined, for example, the vector Sputnik V and Astra Zeneca, but not Sputnik V and Pfizer. It is clear that, as after any medical intervention, complications may arise after vaccination. But to talk about security, we need collective immunity,” says the acting director. Director of the Federal State Budgetary Institution Research Institute of Influenza named after. A. A. Smorodintseva Ministry of Health of Russia Dmitry Lioznov .
Should we wait until the borders open to take advantage of the opportunity to be vaccinated with an imported vaccine? The majority of Russian virologists believe that classical technologies have been tested by time, and therefore are safer than innovative ones.
“There have been no mRNA vaccines at all until now. But when the world faced a pandemic, a powerful impetus was given to the development of this area in order to move to the next level of vaccinology. Huge amounts of money have been invested in such projects, and alliances between startup companies and large biotech companies have begun to emerge in order to ensure the production of the required number of doses. This is a very promising direction, but new and, like any new thing, not without certain risks,” says Corresponding Member of the Russian Academy of Sciences, Director of the Federal Scientific Center for Research and Development named after M.P. Chumakov Aidar Ishmukhametov .
However, any information about vaccines must be treated with caution - it is not easy to separate the struggle of pharmaceutical corporations from medicine and concern for our health.
Author: Natalya Sysoeva
Subscribe to the Invest Foresight channel in Yandex.Zen
WHAT ARE THE WEAKNESSES OF THE VACCINE?
“Sputnik V”: high reactogenicity (that is, the presence of post-vaccination reactions) in people under 60 years of age. According to observations and expert assessments, Sputnik causes fever, pain at the injection site, general weakness, muscle pain, etc. on average, 60% of those vaccinated.
“EpiVacCorona”: leads to the production of specific antibodies, which differ from the antibodies in those who have recovered from the disease and those vaccinated with other vaccines. Confirmation of the protective ability of antibodies after EpiVacCorona in practice is expected.
“CoviVac”: fewer vaccinated people develop antibodies than after “Sputnik”; There is no preliminary data on the effectiveness of the vaccine in practice.
“Sputnik Light”: short-term immune protection - according to the developers, with primary vaccination (that is, not in those who have been ill or previously vaccinated), immunity lasts up to 4-5 months.
Imported analogues of domestic vaccinations for children
Only the mother can decide whether to be vaccinated with Russian vaccines or their foreign analogues. However, before making a final choice, it is worth consulting with a qualified doctor.
From BCG
Babies are given protection against tuberculosis in the first days of life, in the maternity hospital.
The vaccine is valid for approximately seven years, after which it is necessary to vaccinate again.
Today, all over the world, BCG vaccination is done with the same vaccine containing live microorganisms..
Only the manufacturers differ.
Since transportation from other countries is quite long and if its conditions are violated, the death of components intended for introduction into the body and the formation of an immune response may occur, experts recommend giving preference to the Russian vaccine. Another advantage of the latter is that its development took into account the peculiarities of our life, nutrition, children’s genetics, etc.
In Europe, children are not given BCG because the standard of living meets high standards. In Russia, the risk of encountering tuberculosis is tens of times greater.
For hepatitis
The first vaccine against hepatitis B is given to children in the maternity hospital, then two more booster vaccinations are given.
A domestically produced drug is used. You can get a paid vaccination in a private clinic for money.
As a rule, the anti-hepatitis component is included in polyvaccines.
The best are considered: “Infanrix hexa”, “Engerix”. Mono-vaccinations: “Eberbiovak NV”, “Euvax V”, “Shanvak-V”.
The effectiveness of Russian and foreign vaccines against hepatitis is the same, only tolerability, pain, swelling and other unpleasant things differ. The consequences of the introduction after foreign analogues are rare.
DTP
One of the most difficult to tolerate vaccinations protects a child from three dangerous infections at once: whooping cough, diphtheria and tetanus. Reactions occur due to the content of living microorganisms. In addition, the Russian vaccine is poorly tolerated due to the fact that it is made on the basis of aluminum hydroxide and contains a very dangerous component called “merthiolate.”
According to the national schedule, it is carried out at 3, 4.5 and 6 months, with revaccination at one and a half years.
Popular foreign analogues:
- "Infanrix". The weakened pertussis element, polycomponent nature, as well as the absence of merthiolate and formaldehyde in the composition make the drug one of the best existing on the market;
- Pentaxim is a popular French vaccine that protects not only against whooping cough, diphtheria and tetanus, but also Haemophilus influenzae and polio;
- "Tetrakok" - DTP and polio.
If possible, DTP vaccination should be given preference to foreign vaccinations. Severe reactions to them are practically not noted.
From polio
There are two types of vaccines against this infection:
- OPV . Contains a live weakened strain of the virus. Produced in Russia, not used abroad. It is more difficult to tolerate, however, it guarantees more powerful protection against polio;
- IPV . Contains an inactivated strain and is used where the risk of contracting polio is minimal or absent (the Russian Federation does not apply to such territories). This type is represented by foreign vaccines.
Immunization is carried out with the following vaccination material:
- monocomponent – “Poliorix”, “Imovax Polio”;
- multicomponent - “Pentaxim”, “Infanrix Hexa”.
Immunization schedule at an early age: 3, 4.5 and 6 months, with revaccination at 18 years.
If parents have decided to protect their child with paid polyvaccines, then the polio component is already present there; there is no need to give a separate injection.
Measles, mumps and rubella
The following types of immunological drugs for these childhood infections are registered in our country:
- associated divaccine against measles and mumps (manufactured in Russia). Administered simultaneously with a rubella injection (produced in India);
- combined, protecting against three infections at once: MMR-II (USA) and Priorix (Belgium).
Despite approximately the same tolerability, the foreign vaccine is more convenient due to the fact that one injection is administered rather than two. In addition, our immunopreparation is made from quail egg white, which makes it possible to administer to children who are allergic to chicken eggs.
Optional (additional) vaccinations
Since the vaccinations listed below are not included in the national calendar, they can only be done for a fee and on a voluntary basis (that is, by independently contacting a specialized medical center).
Today you can protect yourself from the following infections:
- chicken pox;
- rotavirus infection (intestinal flu, extremely dangerous for children under one year old);
- meningococcal infection;
- human papillomavirus;
- Haemophilus influenzae (causes meningitis, laryngeal edema and other dangerous diseases).
Each parent decides for himself whether to give these vaccines to his child. The listed infections are also extremely dangerous and can lead to death or disability.
FEATURES OF THE VACCINE
Sputnik V: the only Russian vaccine whose safety and effectiveness data have been published in a reputable international peer-reviewed scientific and medical journal (The Lancet). The only Russian vaccine that is approved for use in pregnant women.
“EpiVacCorona”: to check the production of antibodies, a special test is needed (standard tests, suitable for other vaccines, do not recognize antibodies after “EpiVacCorona”). Name of the test system: Set of reagents for the enzyme-linked immunosorbent detection of class G immunoglobulins to the proteins of the SARS-CoV-2 coronavirus “SARS-CoV-2-IgG-Vector”.
CoviVac: The vaccine is based on classic technology that has been used to create vaccinations throughout the history of vaccination (more than 200 years). CoviVac has not yet received official approval for use in people over 60 years of age.
"Sputnik Light": today is not approved for use in persons aged 60 years and older.
The main differences between Russian vaccines and covid.
Photo: Nail VALIULIN
What vaccines can you get vaccinated in Russia?
- "Gum-Covid-Vac" ().
This combined vector vaccine was created by the Center. N. F. Gamaleya and registered in 2021. It is based on neutralized particles of two strains of adenoviruses. Instead of a genetic basis, the drug contains a gene with the coronavirus “spike” code. This protein does not pose a danger to humans, since it does not contain real coronavirus, and helps to produce antibodies.
Vaccination involves two injections with an interval of 21 days. The first one triggers the immune response, the second one strengthens it.
The effectiveness of the vaccine is 91.4%. According to research results, it protects 100% from severe coronavirus. Immunity is expected to develop within 2 years.
- People 18–60 years old, as well as those in the 60+ age category, can get vaccinated.
- After administration, a flu-like syndrome is possible for 1–2 days.
- Contraindications include a history of severe allergic reactions, acute infections, exacerbation of chronic diseases, critical complications after the first dose.
The Sputnik V vaccine in Russia became the first approved for use in people in the 60+ category
- EpiVacCorona.
This vaccine, registered on October 13, 2021, contains small artificially synthesized proteins (peptides) that copy fragments of the coronavirus. In response to them, the body's immune cells produce antibodies. When infected with real Covid-19, the immune system will quickly react to a familiar stimulus.
The vaccine is administered twice with an interval of 14–21 days. After vaccination, immune protection is formed in the body for at least a year. For now, the drug is indicated for people aged 18–60. Estimated immunological effectiveness is 100%.
EpiVacCorona is the second vaccine registered in Russia
- "CoviVac."
In the third decade of February 2021, another vaccine developed by the Center named after. Chumakov RAS. It was created using classical technology - based on inactivated (“killed”) whole coronavirus. It is also administered twice - with an interval of two weeks.
The immunological effectiveness of CoviVac is predicted to be 85%. The vaccine is indicated for people aged 18–60 years. How long immunity will last after immunization is still unknown. Unlike the first two, this drug acts as gently as possible, there are practically no side effects, with the exception of rare cases of pain at the injection site and a short-term increase in body temperature to 38.5 ° C.
“CoviVac” is the “mildest” vaccine in action, which is produced using classical technology
Large-scale mass vaccination against coronavirus in Moscow began on December 5, 2021. At first, vaccinations were available to education and healthcare workers and city social services employees. Then they were joined by representatives of the MFC, the sphere of culture, trade and services, and journalists. On December 26, Sputnik V became available to people over 60 years of age.