Types of influenza vaccines: classification according to WHO and recommendations for use
The World Health Organization divides all existing drugs of biological origin into two types: inactivated vaccines (IIV) and attenuated vaccines (LAIV) [Official source]. They are called trivalent and include strains of three seasonal influenza viruses: pandemic A (H1N1), A (H3N2) and influenza B virus. However, recently, quadrivalent antigen preparations have begun to appear, which already contain 2 strains of the virus from group B, and they can be called a new third type of vaccine.
Influenza vaccination should be carried out annually to obtain the expected effect. Thus, WHO updates the composition of attenuated and inactivated vaccines every year after receiving data on the epidemiological situation from GISRS (Global Influenza Surveillance and Response System).
This organization analyzes various samples of non-cellular infectious agents around the world that are massively infecting people from different countries. After identifying the most dangerous strains, they are added to flu vaccines.
The production of a medical product of biological origin is far from a quick process; it can last 6 months or more [Official source]. The viruses that will be part of the influenza vaccine are cultivated in cell cultures or chicken embryos.
What is the difference between inactivated and attenuated vaccines?
Inactivated influenza vaccines
They are made from microbial particles that have been grown in the laboratory in cell cultures and then destroyed using special poisons or under the influence of high temperatures (heat treatment).
Such antigen preparations can begin to be used from 6 months, but it should be noted that children under 8 years of age who have not received a flu vaccine in the past year must be administered it 2 times with a break of 4 weeks. Inactivated products are also allowed to be used by pregnant women and people with various health conditions and chronic diseases.
This vaccine is injected into the shoulder (deltoid muscle area) or into the thigh muscle area. Intravenous administration is prohibited.
The viruses contained in the inactivated vaccine are not active and do not cause an infectious disease. However, in some cases, adverse reactions occur, for example, pain in various muscles (myalgia), increased body temperature, and a state of general malaise.
Attenuated (live) cold vaccines
The main component of their composition is specially weakened group strains of live viruses. Such vaccines stimulate the immune response at the cellular and local levels.
Attenuated influenza virus vaccines can be administered to children over 2 years of age and adults, but only to people under 50 years of age who do not have health pathologies. Pregnant women and persons with chronic diseases are prohibited from using. Children from 2 to 8 years old who were not vaccinated last season need to be administered an attenuating agent 2 times with a time interval of 1 month.
Side effects of vaccines and who should avoid getting the flu vaccine
As for side effects, they can manifest themselves in the form of nasal congestion, rhinitis (runny nose), pain and sore throat, as well as fever. Side effects are mild compared to the more severe symptoms of colds and flu.
Children under 2 years of age and people aged 50 years and older are most susceptible to such negative consequences. Therefore, it is better for them to refrain from getting the flu vaccine.
Vaccination against influenza in children - how things are going this season
Today, all over the world, the main attention of health authorities, medical workers and all citizens is focused on the new disease COVID-19 (from the English COrona VIrus Disease 2019), caused by the new coronavirus SARS-CoV-2. The epidemic, which emerged in late December 2019 in Hubei Province of the People's Republic of China (PRC), continues to this day with an increasing number of cases and countries involved. On January 30, 2021, the World Health Organization (WHO) designated the novel coronavirus outbreak as a public health emergency of international concern and declared a pandemic on March 11, 2021. It is known that children, including newborns, are involved in the current epidemic process [1, 2]. However, children, while typically more susceptible to colds and influenza than adults, still have lower than expected rates of illness and a milder course of COVID-19. Severe clinical forms of the disease and mortality in children are very rare. According to existing publications, among all cases of COVID-19 registered in China, the proportion of children under the age of 19 was 2.2%; Among confirmed cases of COVID-19 in the United States of America (USA), Italy and Spain, the proportion of children under 18 years of age was 2%, 1.2% and 0.8%, respectively [3–6]. In the Russian Federation, according to the data of the chief freelance specialist of the Ministry of Health of Russia, Academician of the Russian Academy of Sciences Yu. V. Lobzin, announced on April 20, 2020, children with COVID-19 under the age of 18 years are 2.9%, while the level varies in different regions of the country; the absolute majority (90%) of all cases of COVID-19 in children in the Russian Federation are asymptomatic and mild, approximately 9% are of moderate severity, about 1% are severe, but even in such situations, children, as a rule, do not need ventilators and are recovering.
Despite the emergency situation with the pandemic, other diseases, including those of infectious origin, remain relevant. Therefore, along with the unprecedented measures taken throughout the world and in our country to overcome the pandemic, measures to prevent its consequences are important. It is extremely important to continue carrying out full vaccination prophylaxis in children. According to the position of the WHO, the opinion of experts from international professional pediatric organizations and the Union of Pediatricians of Russia, the suspension of the routine immunization process, even for a short time, will lead to an increase in the number of people susceptible to infection and threaten the development of outbreaks and epidemics, for example, measles [7]. In this regard, health services may not be able to cope with the simultaneous workload, already under serious strain due to the pandemic.
Concurrent with the current COVID-19 pandemic, we are also in the middle of the 2019-2020 influenza season, which began in September 2019 and will end in September 2021. Globally, influenza affects 5-10% of adults each year, according to WHO population and 20–30% of children [8]. Moreover, in recent years, WHO experts have repeatedly warned that the threat of pandemic influenza is constantly present and that one thing is clear: another influenza pandemic is inevitable [9]. To protect the population of all countries from this threat, WHO announced the Global Influenza Strategy 2018–2030 in 2021. [10]. The most effective way to protect against influenza and the potentially serious complications caused by influenza infection is to get vaccinated annually. Therefore, one of the main goals of this strategy is to design perfect vaccines and improve the tactics of their use - the transition to quadrivalent drugs, new adjuvants, universal vaccines, and the creation of a collection of strains with pandemic potential.
Many experts agree that influenza poses a great danger along with the new coronavirus infection that has caused a pandemic. In 2021, according to Rospotrebnadzor (Federal Service for Surveillance on Consumer Rights Protection and Human Welfare), 127 cases of death due to influenza were registered, which is 2.7 times higher than in 2021 (46 cases), of which 14 cases among children under 17 years of age (2017 – 13 cases), Fig. 1. The etiological structure of deaths was dominated by influenza caused by the A(H1N1)pdm09 virus.
The death rate from influenza in children in the United States is significantly higher than in the Russian Federation. According to the American Academy of Pediatrics (AAP), 196 children died from influenza in the United States in the 2017–2018 season, and 117 in 2018–2019, half of them previously healthy [12]. Of the children who died with known vaccination status, 80% were not vaccinated. The majority of deaths were observed with influenza A: 43 cases with A(H1N1), 25 with A(H3N2) and 39 with untyped A virus. Eight deaths were associated with B virus. Based on hospitalization data between 2013/2014 and 2016–2017, the cumulative rate of hospitalizations per 100,000 was 72 for children aged 0–4 years and 20 for children aged 5–17 years. Of 1132 hospitalized patients with influenza with relevant data, 45% had premorbid diseases, most often bronchial asthma or obstructive bronchitis (17.7%) and obesity (11.4%).
On April 10, 2020, the US Center for Disease Control and Prevention (CDC) published a report on the 2019–2020 influenza season. in the USA: 166 children have already died from influenza, of which almost two thirds - 104 deaths were associated with influenza B viruses, 25 were determined by origin, all - B/Victoria viruses; 62 deaths were associated with influenza A viruses, 36 were subtypes, 35 were A(H1N1)pdm09 viruses, and one was an A(H3) virus [13]. In the U.S. overall, the CDC estimates that about 39 million people have gotten sick, 400,000 have been hospitalized and 24,000 have died this season.
In the United States, influenza vaccination is not scheduled, but is carried out only in groups at particular risk of high incidence. In this regard, the results of a retrospective study conducted by the American Academy of Pediatrics (AAP) are important [14]. We analyzed 675 deaths in children associated with influenza from 2010 to 2021; the average age of the patients was 6 years. It was shown that half of the deaths from influenza among children occurred in healthy individuals, while only 22% were vaccinated. Nearly two-thirds of children died within seven days of symptom onset. The authors emphasize that only increasing vaccination coverage can reduce childhood mortality associated with influenza.
In the Russian Federation (RF), influenza vaccination is provided free of charge within the framework of the National Calendar to the following groups of the population (Appendix No. 1 to the Order of the Ministry of Health of the Russian Federation
dated March 21, 2014 No. 125n “On approval of the national calendar of preventive vaccinations and the calendar of preventive vaccinations for epidemic indications”):
• Group at high risk of infection:
- children from 6 months;
- students in grades 1–11;
- students studying in professional educational organizations and educational organizations of higher education;
- adults working in certain professions and positions (employees of medical and educational organizations, transport, public utilities);
- pregnant women;
- adults over 60 years of age;
- persons subject to conscription for military service.
• Group at high risk of developing complications after an infection:
- persons with chronic diseases, including lung disease, cardiovascular disease, metabolic disorders and obesity.
In preparation for the epidemic season of influenza and ARVI 2019–2020. To prevent complications of the epidemiological situation regarding influenza and ARVI in the country, Rospotrebnadzor recommended providing vaccination against influenza to at least 45% of the population of a constituent entity of the Russian Federation, including persons from risk groups - at least 75%, provided for by the National calendar of preventive vaccinations [15 ].
In recent years, thanks to the organization of a targeted vaccination campaign and comprehensive efforts to inform the population, the coverage of citizens with influenza vaccination has significantly increased, which has contributed to a steady decline in morbidity. The incidence of influenza in 2021 was 26.33 per 100 thousand population, which is 24.5% lower than the incidence in 2021 - 34.86 per 100 thousand (Fig. 2) [11]. The department has not yet announced the results of 2021.
According to the operational monitoring of Rospotrebnadzor, in 2021, in preparation for the epidemic season, almost 18 million children and more than 46 million adults were vaccinated at the expense of the state. More than 9 million more people have been vaccinated through other sources of funding, including more than 5 million people from employers. In total, more than 73.95 million people were vaccinated, which amounted to 50.5% of the country’s population (www.rospotrebnadzor.ru). At the same time, according to domestic pediatricians, the actual coverage of influenza vaccination among children in the Russian Federation remains insufficient and requires educational work to develop a positive attitude towards immunization among the population [16, 17].
The influenza virus is represented by four groups: A, B, C, D. Moreover, only the first two groups are relevant for humans - A and B. They also cause seasonal epidemics of the disease. The effectiveness of influenza vaccination depends on several factors, such as the age and health of the recipient, the types and subtypes of influenza viruses circulating, and the degree to which circulating viruses are similar to the viruses included in the vaccine.
A feature of the epidemiological situation this season was the active circulation of influenza B viruses, which started from the very beginning of the rise in incidence. According to global influenza monitoring, in the countries of North America, in fact, until the end of December 2019, influenza B viruses were in the lead, and then the circulation of the influenza A (H1N1) virus increased. By the end of March 2021, in many countries of the world, including Russia, the predominance of influenza B viruses was again noted [18]. Typically, influenza B viruses dominate during the decline in incidence, most often in late February and early March. In the current season, they performed dynamically throughout the season: they actively debuted at the beginning and circulated together with the most typical pathogen of influenza A.
Modern influenza vaccines are tri- and quadrivalent preparations that contain current strains of influenza viruses A(H1N1)pdm09, A(H3N2), B/Yamagata-like and/or B/Victoria-like (according to WHO expert recommendations). Every year, the strain composition is reviewed for the countries of the Northern Hemisphere in February-March and for the countries of the Southern Hemisphere in September. According to WHO recommendations on the composition of influenza vaccines for use in the Northern Hemisphere during the 2019–2020 influenza season. both type A viruses at once: a virus similar to A/Michigan/45/2015 (H1N1)pdm09, and A/Singapore/INFIMH-16-0019/2016 (H3N2), which were part of influenza vaccines for use during the 2018–2019 influenza season gg., were replaced by dominant ones this season. The final composition of the influenza vaccine for the 2019–2020 season. included type A viruses: a virus similar to A/Brisbane/02/2018 (H1N1)pdm09, and a virus similar to A/Kansas/14/2017 (H3N2); as well as type B viruses of two genetic lines: a virus similar to B/Colorado/06/2017 (lineage B/Victoria/2/87) and a virus similar to B/Phuket/3073/2013 (lineage B/Yamagata/16/88 ). The first three strains are contained in trivalent vaccines, and the fourth is part of the quadrivalent vaccine.
The range of domestically produced influenza vaccines is currently represented by different types and manufacturers - these are live attenuated intranasal vaccines; inactivated whole virion vaccines; split vaccines; subunit tri- and quadrivalent vaccines (Table 1).
Many studies have confirmed the effectiveness of vaccination in terms of such indicators as a reduction in morbidity by 70–90% in adults and children, the level of hospitalization of elderly people with influenza infection by 21–27%, and the risk of deaths by 12–48% [19]. In the United States, there were six influenza seasons from 2010–2011 to 2015–2016. Influenza vaccination prevented an estimated 1.6–6.7 million illnesses, 790,000–3.1 million outpatient physician visits, 39,000–87,000 hospitalizations, and 3,000–10,000 deaths from respiratory and cardiovascular diseases each season [20]. According to the CDC's weekly report, the vaccine this season overall is 45% effective against seasonal influenza A and B viruses (62% against influenza A(H1N1)pdm09 viruses, 22% against influenza A(H3N2) viruses, and 50% against influenza A(H3N2) viruses. against influenza virus type B). Interim estimates of influenza vaccine effectiveness in children during the 2019–2020 season. amount to 55%, which is comparable to the indicators of previous seasons and provides significant protection for children and adolescents aged 6 months to 18 years [21]. The economic effect of vaccination is 15–20 times higher than the cost of vaccination [20].
In the Russian Federation, in recent seasons, domestic influenza vaccines Grippol® plus, Sovigripp®, Ultrix®, FluM® have been used as part of the National Preventive Vaccination Calendar (Table 2). Since 2017, domestic vaccines Grippol® plus, Sovigripp®, Ultrix® are recommended for children starting from 6 months of age, as well as for pregnant women. Vaccines for children's age groups do not contain a preservative.
In January 2021, the Ministry of Health of the Russian Federation and Rospotrebnadzor approved a plan for the transition until 2023 from the use of trivalent influenza vaccines for immunization of the population within the framework of the National Preventive Vaccination Calendar to the use of quadrivalent ones, since these vaccines significantly increase the effectiveness of immunization against seasonal circulating strains of influenza viruses. According to experts, the quadrivalent influenza vaccine has not only the highest possible efficacy and safety profile to date, but also pharmacoeconomic advantages. Its use will prevent up to 265.8 thousand cases of influenza per season and save more than 2.5 billion rubles. government budget compared to traditional trivalent vaccines.
Also in 2021, the first full-cycle quadrivalent inactivated influenza vaccine of Russian production, Ultrix® Quadri, was registered, which fully complies with WHO recommendations and contains two antigens of influenza virus type A (subtypes A (H1N1) and A (H3N2)) and type B ( Yamagata line and Victoria line) [22, 23]. The vaccine contains 15 mcg of hemagglutinin of each strain in one dose, a total of 60 mcg of antigens per dose. Indications for use of the Ultrix® Quadri vaccine are active annual preventive immunization against seasonal influenza for children from 6 years of age, adolescents and adults up to 60 years of age [23]. It is especially important that the quadrivalent vaccine has become available for vaccination of children, since the epidemic situation with influenza in recent years is characterized by the simultaneous circulation of all 4 strains of influenza viruses - subtypes H1N1 and H3N2 of influenza type A and both Yamagata and Victoria lines of influenza type B, each of which can cause disease. To ensure the implementation of the National Calendar of Preventive Vaccinations, the Russian Ministry of Health purchased 5.4 million doses of the influenza vaccine Ultrix® Quadri for immunization of the adult population from risk groups. It is expected that the effectiveness of this vaccination should be reflected in the incidence of influenza, especially in relation to influenza B viruses, the rate of variation of which is much lower than that of influenza A viruses [22].
It is already known that the recommended composition of the quadrivalent influenza vaccine for the 2020-2021 season, which will begin in September this year, contains type A viruses: a virus like A/Guangdong-Maonan/SWL1536/2019 (H1N1)pdm09 and a virus like A/Hong Kong/2671/2019 (H3N2); as well as type B viruses of two genetic lines: a virus like B/Washington/02/2019 (lineage B/Victoria) and a virus like B/Phuket/3073/2013 (lineage B/Yamagata/16/88).
The COVID-19 situation is an emergency and is changing rapidly. Since this disease is caused by a new virus, we do not yet have immunity to it, and a vaccine is only being developed. Despite the fact that all attention today is focused on the COVID-19 epidemic in the country, immunization against vaccine-preventable diseases is very important. Ensuring routine vaccination in accordance with the National Vaccination Calendar for people of all ages will help avoid even more severe consequences of the new coronavirus infection for the medical care system. COVID-19 and influenza are diseases that claim thousands of lives [24]. Unlike COVID-19, a flu vaccine is available and is effective in preventing complications and reducing the severity of the disease. Adults and children can protect themselves against the flu this coming season by getting vaccinated.
Literature
- Zimmermann P., Curtis N. Coronavirus Infections in Children Including COVID-19: An Overview of the Epidemiology, Clinical Features, Diagnosis, Treatment and Prevention Options in Children // Pediatr Infect Dis J. 2020; 39 (5): 355–368.
- Rasmussen SA, Thompson LA Coronavirus Disease 2021 and Children: What Pediatric Health Care Clinicians Need to Know // JAMA Pediatr. 2020; DOI: 10.1001/jamapediatrics.2020.1224.
- Al-Tawfiq JA, Kattan RF, Memish ZA Middle East respiratory syndrome coronavirus disease is rare in children: An update from Saudi Arabia // World J Clin Pediatr. 2016; 5 (4): 391–396.
- Bartenfeld M. et al. Middle East Respiratory Syndrome Coronavirus and Children // Clin Pediatr (Phila). 2017; 56 (2): 187–189.
- Centers for Disease Control and Prevention. Coronavirus. 2020; Available from: https://www.cdc.gov/coronavirus/2019-ncov/index.html; accessed 6 Mar 2021.
- Chan JF et al. A family cluster of pneumonia associated with the 2021 novel coronavirus indicating person-to-person transmission: a study of a family cluster // Lancet. 2020; 24:24.
- The position of experts of the Union of Pediatricians of Russia regarding vaccination during a pandemic. https://www.pediatr-russia.ru/COVID-19/detail.php?ELEMENT_CODE=vaktsinatsiya-v-period-pandemii.
- https://www.who.int/influenza/ru/.
- https://www.who.int/ru/news-room/feature-stories/detail/8-things-to-know-about-pandemic-influenza.
- World Health Organization. (2019). Global influenza strategy 2019–2030. World Health Organization. https://apps.who.int/iris/handle/10665/311184. License: CC BY-NC-SA 3.0 IGO.
- State report “On the state of sanitary and epidemiological well-being of the population in the Russian Federation in 2021.” https://www.rospotrebnadzor.ru/upload/iblock/798/gosudarstvennyy-doklad-o-sostoyanii-sanitarno_epidemiologicheskogo-blagopoluchiya-naseleniya-v-rossiyskoy-federatsii-v-2018-godu.pdf.
- Committee on Infectious Diseases. Recommendations for Prevention and Control of Influenza in Children, 2019-2020 // Pediatrics. 2019; Sep 2: e20192478. DOI: 10.1542/peds.2019–2478.
- https://www.aappublications.org/news/2020/04/10/fluupdate041020.
- Shang M., Blanton L., Brammer L., Olsen SJ, Fry AM Influenza-associated pediatric deaths in the United States, 2010–2016 // Pediatrics. 2017; 141(4):e20172918. DOI: 10.1542/peds.2017–2918.
- Resolution of the Chief State Sanitary Doctor of the Russian Federation dated July 10, 2021 No. 10 “On measures to prevent influenza and acute respiratory viral infections in the 2019-2020 epidemic season”, Moscow. https://www.rospotrebnadzor.ru/deyatelnost/epidemiological-surveillance/?ELEMENT_ID=12524.
- Tatochenko V.K. Recommendations for the prevention and control of influenza in children for 2019/2020: position of the American Academy of Pediatrics // Issues of modern pediatrics. 2019; 18 (4): 302–304.
- Kramar L.V., Nevinsky A.B. The role of a pediatrician in the formation of parental commitment to vaccinating children against influenza // Children's infections. 2015; 14 (3): 64–67.
- https://www.influenza.spb.ru/system/epidemic_situation/situation_on_a_flu/.
- Popova A. Yu., Ezhlova E. B., Melnikova A. A., Frolova N. V., Mikheev V. N., Ryzhikov A. B. et al. The influence of annual immunization against influenza on the incidence of this infection in the population of the Russian Federation // Epidemiology and vaccine prevention. 2016; 1 (86): 48–55.
- Grohskopf LA, Alyanak E., Broder KR, Walter EB, Fry AM, Jernigan DB Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices - United States, 2019–20 Influenza Season // MMWR Recomm Rep. 2019; 68 (No. RR-3): 1–21. DOI: https://dx.doi.org/10.15585/mmwr.rr6803a1externalicon.
- Dawood FS, Chung JR, Kim SS et al. Interim Estimates of 2019–20 Seasonal Influenza Vaccine Effectiveness - United States, February 2021 // MMWR Morb Mortal Wkly Rep. 2020; 69: 177–182. DOI: https://dx.doi.org/10.15585/mmwr.mm6907a1external icon.
- Briko N.I., Nikiforov V.V., Suranova T.G., Polezhaeva A.N., Saltykova T.S. Immunoprophylaxis and treatment of influenza: successes and problems // Attending Physician. 2019; 12:53–58.
- Change No. 1 to the Instructions for medical use of the drug Ultrix® Quadri, Ministry of Health of Russia, LP-005534-190610 dated 02/13/2020.
- Livingston E., Bucher K., Rekito A. Coronarvirus disease 2019 and influenza // JAMA Network. https://jamanetwork.com/journals/jama/fullarticle/2762386?resultClick=1.
V. A. Bulgakova*, 1, Doctor of Medical Sciences L. R. Selimzyanova***, Candidate of Medical Sciences D. S. Chemakina*, Candidate of Medical Sciences T. E. Privalova**, Candidate of Medical Sciences
* Federal State Autonomous Institution National Medical Research Center for Children's Health, Ministry of Health of the Russian Federation, Moscow ** Federal State Autonomous Educational Institution of Higher Education Russian National Research Medical University named after. N. I. Pirogov Ministry of Health of Russia, Moscow *** Federal State Autonomous Educational Institution of Higher Education First Moscow State Medical University named after. I. M. Sechenov Ministry of Health of Russia, Moscow
1 Contact information
DOI: 10.26295/OS.2020.58.32.010
Vaccination against influenza in children - how are things going this season / V. A. Bulgakova, L. R. Selimzyanova, D. Chemakina, T. E. Privalova For citation: Attending physician No. 5/2020; Page numbers in the issue: 54-58 Tags: children, epidemic process, infection, immunization
Is the vaccine effective against all types of cold and flu viruses?
Seasonal flu vaccines are designed to protect against infections and diseases caused by the three or four flu viruses (depending on the vaccine) that research shows will be most common during the flu season. “Trivalent” influenza vaccines are designed to protect against three influenza viruses, while “tedrivalent” influenza vaccines protect against four influenza viruses. Flu vaccines do not protect against infections and illnesses caused by other viruses, which can also cause flu symptoms . In addition to influenza viruses, there are many other viruses that can cause influenza-like illness (also known as influenza-like illness or “ILI”) that spreads during the flu season. These non-flu viruses include rhinovirus (one cause of the “cold”) and respiratory syncytial virus, which is the most common cause of severe respiratory illness in young children and the leading cause of severe respiratory illness in adults age 65 and older.
Why did imported vaccines disappear?
This year, the French-made vaccine “Vaxigripp” was imported in the same volume as in 2021, according to representatives of the manufacturing company who transferred the products to distributors.
Employees of the Medsi clinic said that they have the French-made vaccine Vaxigripp, but due to the production load on suppliers, only 200 thousand doses were brought to Russia. At the same time, the most popular flu vaccine, produced in Holland, Influvac, was stopped being supplied to Russia altogether. Medsi noted that it “is no longer and will not be.”
I did not import the vaccine into Russia. Officially, the company stated that production was fully loaded and it was not possible to provide additional supplies for Russia. Alexey Yakovlev in his blog connects this with the fact that this year it became unprofitable for foreign suppliers to import vaccines to the Russian market due to the depreciation of the ruble.
The Moscow chain of Chaika clinics does not have foreign vaccines. Instead, she offers the domestic drug Ultrix for vaccination, which, as stated on the website, is similar to Vaxigrip.
Benefits of treating influenza with herbal vaccines
The quadrivalent vaccine with virus-like particles of plant origin was invented not so long ago and has not yet become widely used, but its advantages over other vaccines are already clearly emerging.
No age restrictions
For example, attenuine (live vaccines) are not recommended for use by people over 50 years of age, that is, they cannot get vaccinated once a year to prevent influenza, and to prevent diseases they must constantly take antiviral pills. In a study of people over 65 years of age, the QVLP vaccine [Verified Source] was shown to be effective for use in older adults.
Effective on more viruses
This medical product of biological origin is quadrivalent, which means that its ultimate goal is to develop immunity in the body to 4 types of viruses, two each from group A and B. It should be noted that inactivated and attenuin vaccines are trivalent and can protect only against 2 types of viruses A and one virus from group B.
Does not have foreign impurities
Because QVLP is a plant-derived vaccine [Verified Source] and is unlikely to be contaminated with foreign microorganisms, it is considered purer than trivalent flu medications.
Low percentage of adverse reactions
For people aged 18 to 64 years, the chance of experiencing side effects is 1%, and from age 65 it increases to 4.1%. At the same time, patients who receive attenuin and inactivated vaccines are at higher risk of experiencing flu symptoms.
Should you get a flu shot?
- Yes, if you often have to communicate with people , travel in public transport and be in different public areas, where the risk of contracting the flu is especially high.
- Yes, since vaccination reduces the risk of infection by almost 78%. It is possible that you will get the flu, but it is unlikely that you will get the flu after receiving the vaccine.
- Even if this happens, the disease is tolerated in a mild form and without complications.
- Yes, because any vaccination activates the protective functions of your body.
- You will become stronger and feel better , as it will be easier for your body to resist viral attacks.
Restrictions after vaccination
As a rule, the introduction of vaccines leads to some restrictions regarding lifestyle and the use of foods and medications. When asked what not to do after getting a flu shot, doctors explain:
- supercool;
- overheat (visiting baths and saunas);
- rub the vaccine injection site;
- drink alcoholic drinks.
The optimal time for vaccination is the end of the work week. The process of developing immunity may be accompanied by discomfort in the body, a feeling of weakness, lethargy and drowsiness. The upcoming weekend will provide an opportunity to rest and recuperate.
You should also not forget about following some diet. Young children are not introduced to any new foods for 2 weeks: 7 days before vaccination and 7 days after. This will allow you to evaluate possible allergic manifestations and not confuse them with a reaction to food. For adults, it is enough to limit the consumption of potentially reactogenic products for 1-2 days. You should avoid citrus fruits, nuts, sweet juices and nectars.
Got vaccinated and got sick
Flu vaccines, which are usually administered to the public for prevention, do not contain the virus itself. They themselves cannot cause the disease. But sometimes a person goes for an injection, already in the incubation period of another acute respiratory infection: the causative agent of the disease begins to multiply in the body, but there are no symptoms of the disease yet. During the autumn-winter period, many viruses circulate among people, and the likelihood of infection is high.
In this case, the appearance of acute respiratory infections symptoms: malaise, chills, fever, sore throat or runny nose is completely unconsciously associated with the vaccine. Confusion arises: what to do if you get sick after getting a flu shot?
The right decision would be to contact a therapist for examination and treatment; if you have a high temperature, you should call a doctor at home.
If a person experiences warning signs of acute respiratory viral infection, vaccination should be postponed for 2 weeks.
Flu vaccination 2021-2022: a healthier choice
The annual influenza epidemic cannot be ignored or “moved” in time. The infection usually begins to spread in December. The disease begins to “capture” more and more Russians after the New Year.
A vaccine is a method of specific protection against a dangerous infection, which leads to the formation of immunity after 8-12 days or more. The composition of the vaccine changes every year according to the World Health Organization. According to clinical studies, antibodies are produced in 90% or more of people who receive the vaccine.
Who should delay vaccination?
When considering whether to get a flu vaccine, you need to evaluate possible contraindications to vaccination. They are divided into 2 groups – absolute and temporary.
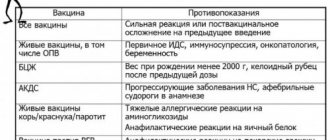
Absolute contraindications are considered allergic reactions to chicken egg white, as well as severe post-vaccination complications or reactions due to a flu shot:
- fever above 40°C;
- swelling or redness with a diameter of 8 cm at the injection site;
- collapse, convulsions;
- anaphylactic shock.
Temporary contraindications indicate the presence of a disease or condition that requires postponing vaccination for some time. These include:
- acute infectious diseases (vaccination is carried out after 2-4 weeks, in some cases earlier);
- chronic diseases in the acute stage (decompensation) - they are vaccinated against influenza after going into remission.
Influenza is dangerous for women during pregnancy. Some vaccines are not recommended for use in this population. Pregnant women are vaccinated in the II-III trimesters of pregnancy with a vaccine without preservatives.
Why is influenza a dangerous and unpredictable virus?
The insidiousness of the influenza virus lies in its ability to quickly mutate in order to once again become unrecognizable to the human immune system. This ability causes new epidemics and pandemics every year, killing thousands of people.
The influenza virus, although considered a controlled infection, remains unique - the ability to constantly change with the emergence of strains with fundamentally new properties makes the flu unpredictable and dangerous, says Nikolai Briko, MD, Professor, Head of the Department of Epidemiology and Evidence-Based Medicine of the First Moscow State Medical University them. I. M. Sechenova, chief freelance epidemiologist of the Russian Ministry of Health, academician of the Russian Academy of Sciences:
— Influenza pandemics have accompanied humanity for many centuries. And today, despite the accumulated knowledge and modern advances in virology, genetics, molecular biology, chemistry and the presence of high-tech industries, the fight against the influenza virus does not weaken, moving to a new stage.
Influenza is not only a severe blow to the immune system, but also the threat of complications: pneumonia, otitis media, sometimes turning into meningitis (inflammation of the membranes of the brain), damage to the cardiovascular and nervous systems. Influenza is especially dangerous for children and expectant mothers, as it can cause disruptions in the course of pregnancy and pathology in the fetus and the elderly. If you have diabetes, asthma or other chronic diseases, you are also at risk.