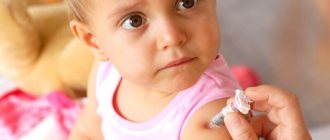
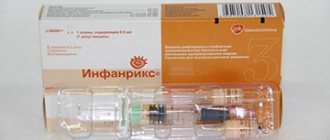
"INFANRIX" - vaccination against diphtheria, tetanus, whooping cough
The vaccine is acellular, three-component, adsorbed, liquid
Manufacturer: GlaxoSmithKline Biologicals, Belgium.
Protects against diseases: diphtheria, tetanus, whooping cough.
For use: in children aged 6 weeks to 7 years.
Included in the national vaccination calendar.
IMPORTANT: vaccinations with the Infanrix vaccine are not carried out in clinics
Composition of the vaccine
The Infanrix vaccine consists of 5 main and 3 additional components. The main ones include:
- diphtheria toxoid1 - not less than 30 IU;
- filamentous hemagglutinin (FHA) - 25 mcg;
- pertussis toxoid (PERT) - 25 mcg;
- tetanus toxoid2 - at least 40 IU;
- pertactin (PRN, outer membrane protein 69 kDa) - 8 mcg.
Additional items include:
- purified water - up to 0.5 ml;
- sodium chloride - 4.5 mg;
- aqueous aluminum oxide3 - 0.5 mg.
1Diphtheria toxoid content 10 Lf (flocculating units);
2Tetanus toxoid content 25 Lf (flocculating units);
3 Conversion to aluminum.
The vaccine does not contain whole cells of the whooping cough pathogen, therefore it is cleaner, safer and less allergenic than the closest analogue of DPT. At the same time, it has been clinically proven that the vaccine forms no less strong immunity than the Russian analogue, and reduces the likelihood of disease in weakened children, which is possible with DTP vaccination.
The drug meets the requirements of the World Health Organization (WHO).
Vaccination is available at our clinic. Sign up for vaccination in advance so that you can get immunized at a time convenient for you.
Contraindications
Only a doctor can decide whether Infanrix is suitable for vaccination
Infanrix vaccination is contraindicated if there is a history of an allergic reaction to any component of the vaccine, as well as in the following cases:
- Hypersensitivity to the active substances or to any excipients.
- Hypersensitivity after previous vaccination against diphtheria, tetanus, whooping cough.
- Case of encephalopathy within 7 days after any pertussis vaccination.
- Progressive neurological disorders.
- Cases of Julian-Barre syndrome within 6 weeks of a previous tetanus vaccination.
DTP_Infanrix
Infanrix is the first vaccine in Russia for the prevention of whooping cough, diphtheria and tetanus with an acellular pertussis component. Combined vaccine based on safe pertussis antigens.
- acellular (cell-free) DTP vaccine;
- 10 years of use worldwide;
- effective and long-lasting protection proven through mass immunization;
- immunogenicity corresponds to the reference DTP vaccine;
- undeniable lower reactogenicity compared to whole-cell DTP vaccines;
- fully complies with the National Vaccination Calendar of Russia.
Pharmacological properties:
Infanrix is a trivalent purified pertussis-diphtheria-tetanus vaccine based on acellular pertussis components (a mixture of three purified antigens of pertussis pathogens), sorbed on aluminum hydroxide. Administration of Infanrix according to the approved vaccination schedule causes the formation of specific immunity against diphtheria, tetanus and whooping cough. The Infanrix vaccine meets WHO requirements for the production of substances of biological origin and vaccines against diphtheria, tetanus and pertussis. The protective effectiveness of the Infanrix vaccine reaches more than 88%. Immune response to primary immunization with the Infanrix vaccine: 1 month after a three-dose course of primary vaccination with Infanrix, carried out in the first 6 months of life, in more than 99% of immunized children, antibody titers to diphtheria and tetanus toxoids are more than 0.1 IU/ml. A secondary immune response to Infanrix pertussis antigens occurs in more than 96% of children. Immune response to revaccination with the Infanrix vaccine: After revaccination with the Infanrix vaccine in the second year of life (13-24 months), in all children who were primarily immunized with the Infanrix vaccine, antibody titers to diphtheria and tetanus toxoids are more than 0.1 IU/ml. A secondary immune response to Infanrix pertussis antigens occurs in more than 96% of children. The Infanrix vaccine practically does not cause anxiety, fever or post-infectious infiltration in the child.
Indications: The Infanrix vaccine is intended for the prevention of diphtheria, tetanus and whooping cough in children:
- primary vaccination against diphtheria, tetanus and whooping cough in children from 3 months;
- revaccination of children who were previously immunized with three doses of pertussis-diphtheria-tetanus vaccine (DTP).
Method of administration and dosage: The Infanrix vaccine is indicated for primary immunization of children, starting from the 6th week of life. Infanrix is administered deeply intramuscularly, alternating injection sites during the course of vaccination. SC and IV administration of Infanrix is contraindicated. The recommended single dose of Infanrix is 0.5 ml. The vaccination course consists of 4 vaccinations. According to the National Calendar of Preventive Vaccinations of Russia, primary immunization with Infanrix begins at 3 months of a child’s life according to the scheme: 3 - 4.5 - 6 months. The Infanrix vaccine is indicated for revaccination of children at 18 months of age if they have previously received three doses of acellular or whole-cell DTP vaccine. Before vaccination, the vial or syringe must be thoroughly shaken until a homogeneous suspension is obtained, making sure there are no foreign impurities or changes in appearance (if any, the Infanrix vaccine is not suitable for use). A vaccine with damaged integrity, lack of labeling, expired or improperly stored is not suitable for use. When using Infanrix in vials, the vaccination procedure is carried out in strict compliance with the rules of asepsis and antiseptics. Infanrix cannot be stored in an open bottle. The administration of Infanrix is recorded in established accounting forms.
Contraindications:
- severe complications that occurred after the previous dose of the Infanrix vaccine (anaphylactic shock);
- allergy to any component of the Infanrix vaccine;
- progressive diseases of the nervous system, hydrocephalus and hydrocephalic syndrome in the stage of decompensation, epilepsy, epileptic syndrome with convulsive seizures;
- anemia with a hemoglobin level below 80 g/l (preventive vaccinations can be carried out after an increase in hemoglobin levels).
Side effects: Infanrix vaccine (with acellular pertussis component) is significantly less reactogenic compared to vaccines containing whole cell pertussis component. Some people vaccinated in the first two days after vaccination with Infanrix may develop short-term general (fever, malaise) and local (pain at the site of vaccine administration, hyperemia, swelling) reactions. Possible complications: convulsions associated with hyperthermia, allergic reactions (Quincke's edema, urticaria, polymorphic rash), exacerbation of chronic diseases. Considering the possibility of developing immediate allergic reactions in sensitive children, it is necessary to provide medical supervision for 30 minutes after vaccination with Infanrix.
Special instructions and precautions: Infanrix vaccine should be administered with caution to persons with thrombocytopenia or hematopoietic dysfunction, since such patients are at risk of bleeding after intramuscular injection. To prevent bleeding, apply pressure to the injection site without rubbing for 2 minutes. Vaccination with Infanrix can be carried out on the same day as the administration of other vaccines according to the vaccination calendar. Infanrix should not be mixed in the same syringe with other vaccines.
Possible side effects
- Local reaction at the injection site (pain, redness, swelling) is not common, ranging from 10% to 53% of vaccination cases. Most often, local reactions are expressed when doses No. 4 and No. 5 are administered.
- General reactions most often include fever, drowsiness, irritability and loss of appetite.
- Premature infants may experience sleep apnea after vaccination.
- In hot weather, vaccinated people may experience cases of loss of consciousness and prolonged high-pitched crying lasting more than 3 hours.
Come get vaccinated at ONNI.
A full range of vaccines for children and adults, family vaccinations - at a special price! Call a doctor at home Make an appointment with a doctor or call +7 (812) 331-17-74
Indications for vaccination
The vaccine is included in the mandatory vaccination schedule, followed by three booster vaccinations. "Infanrix" is used for the primary formation of immunity against tetanus, diphtheria and whooping cough.
The vaccine is also indicated for revaccination of children (according to the plan) previously vaccinated with 3 doses of whole- or acellular vaccination (against tetanus, whooping cough, diphtheria).
Please note: if whole cell vaccines were used first, then acellular vaccines can be used later. It also works the other way around.
As a result, the child’s body produces a sufficient amount of antibodies to resist diseases.
Possible side effects
European vaccines do not contain a preservative, so they rarely cause side effects.
"Infanrix" in children, no more than 0.1% of the number of vaccinated children, can provoke some complications and side effects:
- temperature increase;
- vomit;
- diarrhea;
- pain, redness and/or swelling in the injection area;
- loss of appetite;
- drowsiness;
- weakness;
- allergic reaction up to angioedema and anaphylactic shock.
Side effects may occur within 3 days after vaccination. But experts say it is better tolerated than domestic DTP. If a child has a temperature above 38.5℃ and persists for more than 3 days, this is a reason to seek medical help.
To vaccinate or not?
The issue of childhood vaccinations causes perhaps the most heated debate among modern parents. “Vaccinators” appeal to the fact that vaccination is the only proven way to train the immune system to fight deadly infections, and it is obviously safer than a previous illness. “Anti-vaxxers” point out the unwanted side effects that are possible with vaccination. In fact, blindly following everything that is prescribed at the district clinic and categorically denying all the achievements of modern medicine are equally meaningless. Most parents would still like to make a balanced, informed decision. This means that each specific vaccine should be considered separately.
In this case, we are talking about vaccinating a child under one year old against whooping cough, diphtheria and tetanus. Is vaccination aimed at preventing these diseases justified?
Scheme and method of administration of the Infanrix Hexa vaccine
The vaccine is injected deeply intramuscularly into the middle third of the front surface of the child's thigh. Infanrix Hexa is prescribed as a three-dose primary vaccination in children under 6 months of age. The same dose of the vaccine is administered three times with an interval of 45 days:
at 4.5 – 6 months (recommended by the National Calendar);
How to prepare for Infanrix vaccination
The likelihood of adverse reactions occurring, as well as their severity, can be reduced by preparing for vaccination. First, you need to make sure that the child is healthy. Even a mild ARVI without fever is a temporary contraindication to vaccination. If the child is healthy, but one of the relatives who had contact with the child for several days before vaccination is sick, it is also better to reschedule the vaccination.
It is better to reschedule the vaccination if it is very hot outside or, conversely, very cold. In general, gentle conditions are indicated on the day of vaccination and for several days after. Stressful factors, including walking in the heat and cold, must be excluded.
It is recommended not to overload the stomach on the day of vaccination; vaccination should be done at least an hour after meals. On the day of vaccination, new complementary foods should not be introduced. It is recommended to pay attention to whether the child had bowel movements during the day before vaccination, and if not, give him an enema to help empty his bowels.
On the day the vaccination is scheduled, you can give your child an antihistamine.