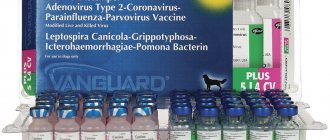
Description of Vanguard 7 Plus
Vanguard 7 vaccine for dogs according to the instructions for use in the package contains 25 doses.
Vanguard 7 is a vaccine product that is a 7-valent vaccine against major infectious pathogens of dogs. Vanguard 7 Plus injection consists of two factions:
- Vanguard DA2Pi:
lyophilized fraction containing live attenuated canine distemper virus strain Snyder Hill = 103.0 CCID50, live attenuated canine adenovirus type 2 Manhattan strain = 103.2 CCID50 and live attenuated parainfluenza virus strain NL-CPI-5 = 106 ,0 CCID50. - Vanguard CPV-L: Liquid fraction containing live attenuated canine parvovirus, strain NL-35-D, low passage = 107.0 CCID50, inactivated Leptospira canicola antigen = PD40 and inactivated Leptospira icterohaemorrhagiae = PD40.
Contraindications, consequences of use
Vanguard is prohibited for use during pregnancy, because Gentamicin has an inhibitory effect on embryos.
In addition, the vaccine is not accepted:
- when feeding;
- in case of painful conditions of the animal;
- in the presence of reactions (allergic) to Vanguard components.
Weak and old dogs are not vaccinated.
The vaccine is well tolerated by puppies and adults without causing side effects. In cases where Vanguard doses were exceeded, no symptoms of infection with coronavirus or plague were recorded. Sometimes, anaphylaxis developed, the consequences of which were treated with the use of symptomatic remedies.
Benefits of using Vanguard 7 for dogs
For active immunization of healthy puppies and dogs to prevent mortality and clinical signs caused by viral infections:
- canine distemper
- parvovirus infections
- adenoviral infections
- Leptospira canicola
- Leptospira icterohaemorrhagiae
- canine parainfluenza
The onset of immunity occurs approximately two weeks after the last dose of Vanguard 7 in the basic vaccination regimen. The duration of immunity to canine distemper virus, canine parvovirus, canine adenovirus types 1 and 2 and leptospiral components is at least 12 months. However, the duration of immunity to canine parainfluenzavirus has not been determined. For similar vaccines it can be about 3 years.
Basic schemes for using the vaccine in puppies and dogs
Vanguard is designed to ensure healthy development and prevent pathological changes in dogs caused by coronavirus and a number of dangerous diseases.
When vaccinating puppies, the following schemes are used:
- the first time at 6 weeks, the second at 9 weeks, the third at 12. The presented classic combination allows you to develop immunity after a second injection, and the third injection strengthens it. Early vaccination is recommended in tense epidemiological situations;
- vaccination at 9 weeks, and then at 11 - 12. The scheme is relevant for pets whose use was postponed for objective reasons;
- at 12 weeks, then at 16.
In adult dogs, Vanguard is used as follows:
- an adult animal that has never been vaccinated is treated twice. They give the first injection, maintain an interval of a week or two, repeat the injection;
- during revaccination, one dose of Vanguard is administered annually.
For puppies and adult dogs, take the same dosage of 1 ml, regardless of breed and weight. Compliance with the vaccination schedule guarantees 100% protection of the animal from deadly viruses.
Basic vaccination scheme with Vanguard 7:
- Puppies younger than 10 weeks: two doses of Vanguard 7 at least 14 days apart. The first dose can be given at 7 weeks of age.
- The second dose should not be given until at least 10 weeks.
- Puppies 10 weeks of age and older: a single dose of Vanguard 7 followed by a single dose of Vanguard Lepto ci at least 14 days later.
- Booster vaccination: annually.
- Leptospiral components - annual re-vaccination with complex Vanguard series vaccines is recommended.
- Viral components - annual booster vaccination is recommended.
However, if veterinary practitioners are conducting a risk-benefit analysis for individual animals to determine revaccination frequency with Vanguard 7, they should be aware of the following information. Serological data have shown that most dogs, when given at least the first annual booster, can maintain protective levels of immunity to Vanguard 7 viral components for up to 4 years. For more information, please contact the manufacturer.
Vanguard is a vaccine against canine distemper, as well as adenovirus, parainfluenza and parvovirus.
This drug is a modified live virus vaccine.
Instructions for use of the Vanguard Plus vaccine in dogs.
- General instructions. Vaccination of healthy dogs is recommended. Aseptically rehydrate the lyophilized vaccine with the sterile diluent provided, shake well and administer in a dose of 1 ml subcutaneously or intramuscularly.
- Primary vaccination. Healthy dogs 6 weeks of age and older should receive 3 doses, each administered 3 weeks apart.
- Revaccination. An annual booster dose with a single dose is recommended, although, according to the recommendations of the American Veterinary Medical Association and its Council on Biological and Therapeutic Agents, the treating veterinarian should determine the frequency of booster shots based on the animal's lifestyle and risk of exposure to pathogens in that area.
Contraindications, warnings for the use of the Vanguard Seven vaccine
Do not vaccinate sick or pregnant animals.
Vaccinated dogs may have transient local swelling 4-6 hours after vaccination, which resolves in approximately 7 days. If a systemic anaphylactic reaction occurs (eg, vomiting, facial swelling, collapse), administer epinephrine or equivalents.
Sometimes transient swelling may occur at the injection site after an overdose vaccination. In most cases of overdose, treatment is not required. However, if a systemic anaphylactic reaction (eg, vomiting) occurs, epinephrine or similar drugs are administered.
Own vaccine strains of adenovirus type 2 and canine parvovirus of the Vanguard 7 vaccine can be isolated from vaccinated animals within a few days after vaccination. However, due to the low pathogenicity of these strains, there is no need to keep vaccinated animals separately from unvaccinated animals.
High levels of maternally derived antibodies (MDA) may influence the response to vaccination. Although the vaccine has been shown to be effective at levels of MDA likely to be encountered in field settings where particularly high levels of MDA are likely to be present for any reason (eg against the CPV component), this should be taken into account when planning the timing of vaccinations.
SAFETY AND EFFECTIVENESS OF VANGUARD VACCINE FOR DOGS
Laboratory evaluation showed that Vanguard Plus was effective in preventing CD, ICH, CAV-2 respiratory disease, CPI, and CPV and that there were no significant immunologic biases between different vaccine fractions. Extensive field safety testing conducted by Zoetis Inc. has shown Vanguard to be safe with minimal reactions in dogs 6 weeks of age under normal conditions of use.
The CAV-2 vaccine has been demonstrated to cross-protect against ICH caused by CAV-1. In addition, the CAV-2 strain used in Vanguard vaccines was specifically selected to lack the oncogenic properties associated with adenoviruses. Research from Zoetis Inc. has shown that the CAV-2 strain used in Vanguard vaccines protects not only against ICH but also against CAV-2 respiratory disease. Although conventional CAV-1 (ICH) vaccines cross-protect against CAV-2, they may not prevent subclinical infection and spread of the CAV-2 agent. The virus that causes canine adenovirus type 2 was not detected in dogs vaccinated with CAV-2 in tests conducted at Zoetis Inc studying the effectiveness of the Vanguard vaccine.
CPV fraction in the Vanguard Plus vaccine
has been subjected to extensive safety and effectiveness testing by Zoetis Inc. It has been shown to be safe with minimal reactions in laboratory tests and in clinical field trials. The safety of the product was further demonstrated through a challenge-challenge study that involved oral administration of multiple doses of the vaccine strain to susceptible dogs, all of which remained healthy. The CPV virus in Vanguard Plus shares a common characteristic with other live CPV vaccine strains in that the vaccine virus may be present in the stool after administration. Although this CPV vaccine virus has occasionally been detected at low titres in the feces of vaccinated dogs, testing has shown that major vaccine isolates do not return to virulence after 6 consecutive back-passes in susceptible dogs.
Research at Zoetis Inc. showed that 3 doses of Vanguard Plus vaccine with elevated CPV titers could overcome serum neutralization (SN) titers associated with maternal antibody. Serum neutralization titers of up to 1:4 have been shown by others to affect active immunization using conventional modified live vaccines. A clinical trial was conducted on fifty 6-week-old puppies [25 vaccinated (SN titre range less than 2-256) and 25 unvaccinated controls (SN titer range 4-1024)]. The vaccinated group received 3 doses, and vaccination was carried out at an interval of 3 weeks, starting at 6 weeks of age. After 1 Vanguard vaccination, 13/25 puppies experienced a 4-fold or greater increase in SN CPV titer (seroconversion). Twelve of these 13 puppies had maternal SN titers ≤1:16 at the time of the first vaccination, and the remaining pup had an SN titer of 1:64. Another 9 puppies with initial SN titers between 1:16 and 1:256 seroconverted after the second Vanguard vaccination.
Their SN maternal antibody titers decreased to ≤1:64 at the time of the second vaccination. Similarly, the last 3 vaccinees with initial SN titers of 1:128 seroconverted after the third vaccination after their maternal antibody CPV titer had decreased to ≤1:64. Thus, in this study, when 3 vaccine doses were given starting at 6 weeks of age , all 25 vaccinees, even those with the highest levels of maternal antibodies, became actively immunized (GM = 1:1176; SN titer range 128-4096). All 50 dogs were challenged 3 weeks after the third vaccination with heterologous CPV challenge virus. Fourteen of the 25 unvaccinated control dogs died or developed illness severe enough to warrant euthanasia, while all 25 vaccinated remained essentially healthy. Therefore, the high-titer, low-passage vaccine virus in Vanguard Plus 5 is highly immunogenic and is capable of stimulating active immunity in the presence of maternal antibodies.
Pharmaceutical Precautions
Store Vanguard 7 and transport at a temperature of 2–8 ° C.
Do not freeze. There is no information available about the safety and effectiveness of using this vaccine concomitantly with any other vaccine. Therefore, it is recommended that you do not administer any other vaccine within 14 days before or after vaccination with this product. Do not mix with any other vaccine or immunological product.
Dispose of Vanguard 7 waste by boiling, burning or immersing in an appropriate disinfectant in accordance with national requirements. Only for the treatment and prevention of animal diseases. Keep out of the reach of children.
How to administer the vaccine correctly
When carrying out vaccination, attention is paid to three aspects: preparation of the Vanguard dose, injection site, sterility of the instruments used.
Vanguard is prepared immediately before administration. For this:
- shake the bottle with the liquid component slightly;
- pump out the contents with a syringe, after wiping the surface of the lid with a disinfectant solution;
- introduce the solution into the container with the dry component;
- shake until the substances are completely dissolved.
Draw the resulting suspension into a syringe. The injection is given intramuscularly, more commonly – subcutaneously. The site where avant-garde is introduced is treated with alcohol (70%). With a subcutaneous injection, the fold is pulled back and vaccination is performed.
Be sure to adhere to the rules of asepsis and antiseptics that prevent infection from entering the body of an animal or a person, or aimed at its destruction. For this:
Always use disposable syringes, which are treated with special solutions and disposed of. When vaccinating two or more dogs, the procedure is similar. The person administering the vaccine must use rubber gloves that can prevent contact of the suspension components with the skin. It is important to have access to a first aid kit. Avoid contact of the vaccine with mucous membranes. Vanguard's solution is stored for no more than 30 minutes, then it is disinfected by boiling (10 - 15 minutes) or treated with an alkali solution (2%). Recycled.
Further information
The CAV-2 strain used in Vanguard 7 has not been associated in any laboratory
, nor in field studies with corneal opacity (“blue eye”) or adenovirus type 1 (CAV-1)-associated uveitis. Canine parvovirus disease in kennels and pet stores is a problem in intensive livestock production and should be treated as a major concern. Without careful professional attention to management practices in such locations, successful disease control may be difficult to achieve.
When used in accordance with the recommended vaccination regimen, Vanguard 7 may help reduce environmental contamination of canine parvovirus.
NB: Vanguard 7 is a medicine for dogs only and is available on prescription from a veterinarian.
Learn about essential drugs in animals
- Vaccination of puppies
- Vaccination of cats against rabies
- Vaccination of small animals
- Kitten vaccination
- Vaccination of dogs against rabies
- Why do kittens need to be vaccinated?
- Nobivac Triquet Trio
- Nobivac DHPPi for dogs
- Puppy's first vaccination
- Comprehensive vaccinations for cats
- Tick vaccination for dogs
- What vaccinations are given to dogs and why?
- Why is Ursofalk prescribed to cats?
- Instructions for using serenia for animals
- Ursofalk in dogs
- Marfloxin for veterinary medicine
- Instructions for the use of serenia in dogs
- Instructions for using serenia in cats
- Why is Heptor prescribed to a dog?
- Instructions for the use of lidocaine in dogs
- Heptor in cats
- Lidocaine for cats. Can cats use lidocaine?
- Heptral in dogs
- Can cats use marfloxin?
- Marfloxin for dogs (instructions for use)
- Heptral in cats (instructions for use, pharmacology
- Instructions for use of Dirofen for cats
- Instructions for using Vetom in cats
- Instructions: multifel for cats
- Use of fosprenil in cats
^Top