Contraindications
Do not use water for injection as a solvent for medicinal and diagnostic drugs if another solvent is indicated in the instructions for medical use of the drug.
Do not use the drug as an eye wash during ophthalmological operations.
Interaction with other drugs and other types of interactions.
Water for injection does not exhibit pharmacological or chemical interaction with drugs and diagnostic agents intended for subcutaneous, intramuscular or intravenous administration.
What is water for injection
Liquid for injection can be used as a carrier of the main drug (parenteral use) or as a diluting agent for infusion and injection solutions with inappropriate concentrations. Water is produced in the form of glass or polymer fiber ampoules of various filling volumes. Intended, among other things, for external use: wetting dressings, washing wounds and mucous membranes. Medical instruments are soaked and washed in injection water during the sterilization process.
Compound
Sterile water has no taste, color or smell. In a special way, the composition of water for injection is purified from all inclusions: gases, salts, biological components, as well as any microimpurities. This is achieved in two stages. The first is purification by reverse osmosis, during which organic inclusions are separated from water. The second is distillation: the liquid is converted into a vapor state and then returned to its original form. In this way, maximum purity is achieved. Injection water does not have pharmacological activity.
Indications
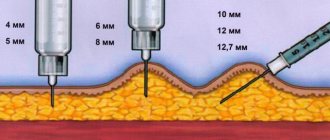
Used for the preparation of sterile injection solutions from dry matter (powders, concentrates, lyophilisates). Can be used to prepare infusions for subcutaneous, intravenous and intramuscular administration. The dosage and method of administration are determined by the drug to be diluted (the manufacturer prescribes these features in the instructions for the drug). The only universal rule is that water must be used under aseptic conditions from the moment the ampoule is opened until the syringes are filled.
Contraindications
Although water is considered a universal solvent, there are preparations that involve the use of another type of liquid. For example, saline solution, oil solvents, etc. These kinds of features must be prescribed in the instructions for the diluted medicinal product. The injection liquid cannot be mixed with preparations for external use, since they use a different type of solvent.
Application Features
Water for injection is intended only for the preparation of sterile solutions of medicinal and diagnostic agents intended for subcutaneous, intramuscular or intravenous administration, the method of application of which involves the use of water for injection.
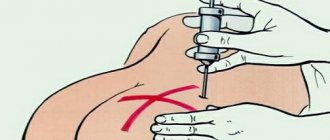
Water for injection cannot be directly administered intravascularly due to low osmotic pressure due to the risk of hemolysis!
Use during pregnancy or breastfeeding.
Can be used during pregnancy or breastfeeding.
The ability to influence the reaction rate when driving vehicles or other mechanisms.
Does not affect.
Requirements for water for injection
The pH value of injection water should not be higher than 5.0-7.0. The concentration of microorganisms in 1 ml is no more than 100. It must be pyrogen-free (devoid of substances that cause an increase in temperature when injecting liquid into the body), with a normalized ammonia content. In water that meets the requirements, the presence of sulfates, chlorides, heavy metals, calcium, nitrates, carbon dioxide and reducing substances is unacceptable.
- How to treat osteoporosis
- Sterilizer for manicure instruments
- Hair tinting at home, photos and videos
What is a distillate?
Distillate is water that is almost completely purified from salts and other organic substances.
This type of mixture is characterized by the following:
low specific electrical conductivity (poorly conducts electric current or does not conduct it at all);- slightly acidic environment (presence of carbon dioxide);
- the beginning of boiling at a temperature exceeding 100C;
- the onset of freezing at temperatures well below 0C (turns into ice at temperatures of -10C and below).
Organic matter has not been completely removed from distilled water. It contains gases. There, oxygen, carbon, as well as nitrogen with argon and a small amount of other substances are present in small concentrations.
FS.2.2.0019.15 Water for injections
H2O M. m. 18.02
This pharmacopoeial monograph applies to bulk water for injection obtained from drinking water by distillation, ion exchange, reverse osmosis, a combination of these methods or other methods, or from water purified by distillation, and intended for the production or manufacture of parenteral and other medicines.
When using water for injection in the technology of parenteral and other drugs obtained immediately before use, under conditions that preclude subsequent sterilization of drugs, water for injection must be sterile.
Water for injections must be pyrogen-free and must not contain antimicrobial preservatives or other additives.
Description
Colorless, transparent, odorless liquid.
pH
From 5.0 to 7.0 (OFS “Ionometry”, method 3). To 100 ml of purified water add 0.3 ml of a saturated solution of potassium chloride.
Acidity or alkalinity
To 20 ml of water for injection add 0.05 ml of 0.1% phenol red solution. When a yellow color appears, it should change to red by adding no more than 0.1 ml of 0.01 M sodium hydroxide solution. When a red color appears, it should change to yellow by adding no more than 0.15 ml of a 0.01 M hydrochloric acid solution.
Electrical conductivity
The determination is carried out in accordance with the General Pharmacopoeia Monograph “Electrical Conductivity” using equipment - conductometers included in the State Register of Measuring Instruments.
Equipment
Conductivity cell:
- electrodes made of a suitable material
such as stainless steel;
— The cell constant
is usually set by the supplier and subsequently verified at appropriate intervals using a certified standard solution with a conductivity of less than 1500 µS/cm or by comparison with a cell having a certified cell constant. The cell constant is considered confirmed if the value found is within 2% of the value specified in the certificate; otherwise, recalibration must be performed.
Conductivity meter. The measurement accuracy must be at least 0.1 µS/cm in the lowest range.
Calibration of the system (conductivity cell and conductivity meter). Calibration must be carried out using one or more appropriate standard solutions (OFS “Electrical Conductivity”). The permissible deviation should be no more than 3% of the measured electrical conductivity value.
Conductivity meter calibration. The conductivity meter is calibrated using high precision resistors or an equivalent device after disconnecting the conductivity cell for all intervals used to measure conductivity and calibrate the cell, with an error of no more than 0.1% of the certified value.
If it is not possible to disconnect a conductivity cell installed in the production line, calibration can be performed relative to a pre-calibrated conductivity cell placed in a stream of water next to the cell being calibrated.
Methodology
Stage 1
Measure electrical conductivity without temperature compensation while simultaneously recording temperature. Conductivity measurements using temperature-compensated conductometers are only possible after appropriate validation.
Find the nearest temperature value (Table 1), less than the measured one. The corresponding value of electrical conductivity is the maximum permissible.
Water for injection meets the requirements if the measured electrical conductivity value does not exceed that found from the table. 1 maximum permissible value.
Table 1. Maximum permissible values of electrical conductivity of water for injection depending on temperature
| Temperature, ºС | Electrical conductivity, µS/cm | Temperature, ºС | Electrical conductivity, µS/cm |
| 0 | 0,6 | 55 | 2,1 |
| 5 | 0,8 | 60 | 2,2 |
| 10 | 0,9 | 65 | 2,4 |
| 15 | 1,0 | 70 | 2,5 |
| 20 | 1,1 | 75 | 2,7 |
| 25 | 1,3 | 80 | 2,7 |
| 30 | 1,4 | 85 | 2,7 |
| 35 | 1,5 | 90 | 2,7 |
| 40 | 1,7 | 95 | 2,9 |
| 45 | 1,8 | 100 | 3,1 |
| 50 | 1,9 |
For temperature values not presented in table. 1, calculate the maximum permissible value of electrical conductivity by interpolating the closest upper and lower values given in table. 1.
If the electrical conductivity value exceeds that given in the table.
1 value, continue testing in accordance with the requirements of stage 2. Stage 2
At least 100 ml of water for injection is placed in the vessel and mixed. With constant stirring, set the temperature within 25 ± 1 ºС and measure the electrical conductivity every 5 minutes until the change in electrical conductivity over 5 minutes is less than 0.1 µS/cm. This electrical conductivity value is recorded.
Water for injection meets the requirements if the obtained electrical conductivity value is no more than 2.1 µS/cm.
If the conductivity value is more than 2.1 µS/cm, test in accordance with the requirements of stage 3.
Stage 3
The test is carried out for approximately 5 minutes after the test in step 2, maintaining the temperature within
25 ± 1 ºС. Add a freshly prepared saturated solution of potassium chloride to water for injection (0.3 ml per 100 ml of water for injection) and determine the pH to the nearest 0.1.
Determine the limiting value of electrical conductivity (Table 2) for a given pH.
Water for injection meets the requirements for electrical conductivity if the electrical conductivity value obtained at stage 2 does not exceed the value given in table. 2. If the electrical conductivity value obtained at stage 2 exceeds the value given in table. 2, or the pH value is outside the range of 5.0–7.0, then water for injection does not meet the requirements for the “Electrical conductivity” indicator.
Table 2. Maximum permissible values of electrical conductivity of water for injection depending on pH
| pH | Electrical conductivity, µS/cm | pH | Electrical conductivity, µS/cm |
| 5,0 | 4,7 | 6,1 | 2,4 |
| 5,1 | 4,1 | 6,2 | 2,5 |
| 5,2 | 3,6 | 6,3 | 2,4 |
| 5,3 | 3,3 | 6,4 | 2,3 |
| 5,4 | 3,0 | 6,5 | 2,2 |
| 5,5 | 2,8 | 6,6 | 2,1 |
| 5,6 | 2,6 | 6,7 | 2,6 |
| 5,7 | 2,5 | 6,8 | 3,1 |
| 5,8 | 2,4 | 6,9 | 3,8 |
| 5,9 | 2,4 | 7,0 | 4,6 |
| 6,0 | 2,4 |
Dry residue
No more than 0.001%. 100 ml of water for injection is evaporated to dryness and dried at a temperature of 100 to 105ºC to constant weight.
Reducing agents
100 ml of water for injection is brought to a boil, 0.1 ml of 0.02 M solution of potassium permanganate and 2 ml of sulfuric acid diluted 16% are added, boil for 10 minutes; The pink color should remain.
Carbon dioxide
When shaking water for injection with an equal volume of calcium hydroxide solution (lime water) in a filled to the top and well-closed container, there should be no turbidity within 1 hour.
Nitrates and nitrites
To 5 ml of water for injection, carefully add 0.1 ml of freshly prepared diphenylamine solution; No blue color should appear.
Ammonium
No more than 0.00002% (ODS “Ammonia”). The determination is carried out using a standard solution containing 1 ml of a standard ammonium ion solution (2 μg/ml) and 9 ml of ammonia-free water. For determination, 10 ml of the test sample is taken.
Note. Ammonium ion standard solution (2 µg/ml) is prepared by diluting ammonium ion standard solution (200 µg/ml) with ammonia-free water.
Chlorides
To 10 ml of water for injection add 0.5 ml of nitric acid, 0.5 ml of 2% silver nitrate solution, mix and leave for 5 minutes. There should be no opalescence.
Sulfates
To 10 ml of water for injection add 0.5 ml of diluted hydrochloric acid 8.3% and 0.1 ml of 5% barium chloride solution, mix and leave for 10 minutes. There should be no cloudiness.
Calcium and magnesium
To 100 ml of water for injection add 2 ml of ammonium chloride buffer solution, pH 10.0, 50 mg of indicator mixture of mordant black 11 and 0.5 ml of 0.01 M sodium edetate solution; A pure blue color of the solution should be observed (without a violet tint).
Aluminum
No more than 0.000001% (OFS “Aluminium”, method 1).
Test solution. To 400 ml of purified water add 10 ml of acetate buffer solution, pH 6.0 and 100 ml of distilled water, mix.
Standard solution. To 2 ml of a standard solution of aluminum ion (2 μg/ml), add 10 ml of acetate buffer solution, pH 6.0 and 98 ml of distilled water, mix.
Control solution. To 10 ml of acetate buffer solution, pH 6.0, add 100 ml of distilled water and mix.
Heavy metals
No more than 0.00001%.
The determination is carried out using one of the given methods.
Method 1. Place 10 ml of test water for injection into a test tube with a diameter of about 1.5 cm, add 1 ml of acetic acid diluted 30%, 2 drops of 2% sodium sulfide solution and mix. After 1 min. Observe the color of the solution along the axis of the test tube placed on a white surface. There should be no staining.
Method 2. 120 ml of water for injection is evaporated to a volume of 20 ml. The remaining water after evaporation in a volume of 10 ml must withstand the test for heavy metals (OPS “Heavy Metals”) using a standard solution containing 1 ml of a standard solution of lead ion (5 μg/ml) and 9 ml of test water for injection.
Note. Lead ion standard solution (5 µg/ml) is prepared by diluting lead ion standard solution (100 µg/ml) with test water for injection.
Microbiological purity
The total number of aerobic microorganisms (bacteria and fungi) is no more than 10 CFU per 100 ml. The presence of Escherichiacoli, Staphylococcusaureus, Pseudomonasaeruginosa in 100 ml is not allowed.
To analyze the microbiological purity of water for injection, a sample in a volume of at least 1000 ml is taken.
The study is carried out by membrane filtration under aseptic conditions in accordance with the methods of the General Pharmacopoeia Monograph “Microbiological purity”, clause 12.
Bacterial endotoxins
Less than 0.25 EU/ml (General Pharmacopoeia Monograph “Bacterial endotoxins”).
Storage and distribution
Water for injections is stored and distributed under conditions that prevent the growth of microorganisms and exclude the possibility of any other contamination.
Water for injection is stored in special containers under the condition of constant circulation at a temperature not lower than 85 ºС for no more than 1 day.
Interchangeability
Both types are not interchangeable. It is not allowed to replace a distillate with a solution that was originally intended to dilute a specific type of medicine.
Distilled water is not completely free of impurities.
Its addition as a solvent for a number of drugs is a gross violation of technology. This mixture must not be used for treatment as it is non-sterile.
Water for injection has a rather narrow application. This composition could potentially be used instead of purified water, but due to the technological thoroughness of production, it is used only for specific purposes.
The uses of distilled water are much wider. But using it to dissolve a certain type of medicine is strictly prohibited.
How is it different from boiled water?
Boiled water is essentially water heated to 100°C. The resulting vapors do not collect or condense. Nothing is done with them.
People usually boil water to kill the germs in it. But unlike distilled water, water is not separated from other trace elements and salts. In fact, they are simply boiled together.
Thus, microorganisms may die, but trace elements and particles are still present, so the water is still not completely pure.
Cases when only one or the other is needed
Distilled solutions are used in the manufacture of non-sterile medicines. These can be various syrups and tinctures. They are also used to prepare liquid products for treating leather. However, such compositions should not come into contact with skin that has wounds .
Distilled water is used in the manufacture of tablets, capsules, powders, sprays and medicinal suppositories. This composition is also used in the production of ointments for treating skin that does not have wounds.
The distillate is also used in the production of sterile drugs. These may include rinses, eye drops, and ointments.
For the manufacture of solutions intended for injection, only a sterile aqueous base is used. Distilled mixtures are not used in such cases.
All the most important and useful information about distilled water is in this section.