Home | About us | Delivery | Advertisers | Login | Registration
- Medicines
- dietary supplementsVitamins
- Categories from A to Z
- Brands from A to Z
- Products from A to Z
- Medical equipment
- beauty
- Child
- Care
- Honey products appointments
- Herbs and herbal teas
- Medical nutrition
- Journey
- Making medicinesStock
Pharmacy online is the best pharmacy in Almaty, delivering medicines to Almaty. An online pharmacy or online pharmacy provides the following types of services: delivery of medicines, medicines to your home. Online pharmacy Almaty or online pharmacy Almaty delivers medicines to your home, as well as home delivery of medicines in Almaty.
my basket
Apteka84.kz is an online pharmacy that offers its customers medicines, medicinal and decorative cosmetics, dietary supplements, vitamins, baby food, intimate products for adults, medical equipment and thousands of other medical and cosmetic products at low prices. All data presented on the Apteka84.kz website is for informational purposes only and is not a substitute for professional medical care. Apteka84.kz strongly recommends that you carefully read the instructions for use contained in each package of medicines and other products. If you currently have any symptoms of the disease, you should seek help from a doctor. You should always tell your doctor or pharmacist about all the medicines you take. If you feel you need further help, please consult your local pharmacist or contact our GP online or by telephone.
© 2021 Pharmacy 84.
Cerebrolysin injection solution ampoules 10ml No. 5
From the immune system: very rarely - increased individual sensitivity, allergic reactions. Mental disorders: rarely - the supposed activation effect is accompanied by agitation, manifested by aggressive behavior, confusion, and insomnia. From the nervous system: rarely - too rapid administration of the drug can lead to dizziness; very rarely - isolated cases of generalized epilepsy and one case of seizures were associated with Cerebrolysin. From the cardiovascular system: very rarely - too rapid administration of the drug can lead to increased heart rate and arrhythmia. From the digestive system: very rarely - dyspepsia, diarrhea, constipation, nausea, vomiting; rarely - loss of appetite. From the skin and subcutaneous tissues: very rarely - skin reactions; rarely - with excessively rapid administration, a feeling of heat, sweating, and itching may occur. General disorders and disorders at the injection site: very rarely - redness, itching, burning at the injection site, pain in the neck, head and limbs, fever, mild back pain, shortness of breath, chills, collapsed state. One study reported an association between the use of the drug in rare cases with hyperventilation, hypertension, hypotension, fatigue, tremor, possible development of depression, apathy and/or drowsiness, and flu-like symptoms (cold, cough, respiratory tract infections). Since Cerebrolysin® is used mainly in elderly patients, the above symptoms of diseases are typical for this age group and often also occur without taking the drug. It should be noted that some undesirable effects (excitement, arterial hypertension, arterial hypotension, lethargy, tremor, depression, apathy, dizziness, headache, shortness of breath, diarrhea, nausea) were identified during clinical studies and occurred equally in patients treated with Cerebrolysin® and in patients in the placebo group. If any of the side effects indicated in the instructions are aggravated or any other side effects not specified in the instructions are noted, the patient should inform the attending physician. Notification of Suspected Side Effects It is important to report side effects after drug approval to ensure ongoing monitoring of the drug's risk-benefit profile. Healthcare professionals are asked to report all cases of adverse reactions observed with the drug through national adverse reaction reporting systems and/or to the company's representative office.
Material and methods
This single-center, randomized, placebo-controlled clinical trial in 2 parallel groups was conducted from March 2015 to January 2021 at the State Autonomous Healthcare Institution “Republican Clinical Neurological”. A total of 40 patients with relapsing-remitting MS were examined (the diagnosis was established in accordance with the MacDonald criteria, 2010), who were undergoing dispensary observation at the Republican Clinical Diagnostic Center for Demyelinating Diseases of the Ministry of Health of the Republic of Tatarstan (RKDC DZ MZ RT). All patients previously signed informed consent to participate in this clinical study. In accordance with the inclusion criteria, patients were recruited regardless of the first-line treatment with MS course-modifying drugs (MSDs), who were in the stage of regression of exacerbation of MS after pulse therapy with methylprednisolone (5 intravenous injections of 1000 mg per day over the last 4 weeks). . The study did not include patients with primary or secondary progressive MS, the presence of any clinically significant pathology and a condition that prevented NFO and brain MRI. Using a software random number generator, patients were randomized into two groups comparable in terms of main clinical and demographic indicators. Patients of group 1 ( n
=20) received Cerebrolysin 20 ml per 200 ml of 0.9% sodium chloride solution intravenously for 10 days.
In group 2 ( n
= 20), patients received placebo in the form of sodium chloride solution according to a similar treatment regimen.
All patients underwent clinical and laboratory examination before the start of treatment and 3-4 weeks after its completion. The clinical examination included a neurological examination with assessment of the condition on the EDSS scale using the original online EDSS calculator (https://edss.ru, registration certificate No. 2016610500). A comprehensive assessment of the functional abilities of MS patients (MSFC scale), a symbolic-digit modality matching test (SDMT scale), and an assessment of visual acuity according to the Snellen chart (LCAT) were carried out. In addition, patients underwent complex NFO with registration of VEP (P100 latency was assessed), MR (R1 latency was assessed), TCMS (LTP and central motor time latencies were assessed), SSEP (P20 latency was assessed, the duration of the N13-N20 interpeak interval ). Significant changes in neurophysiological parameters obtained during repeated studies were considered to be differences from the initial values of 5% or more.
To assess the safety of treatment, 4 patients of group 1 with the presence of previously identified contrast-enhancing lesions (CFs) underwent brain MRI 3-4 weeks after treatment to assess the dynamics of the number of CFs.
Statistical analysis of the obtained data was carried out using the Statistica 10 package after a pre-prepared table of primary data in the Excel application from the Microsoft Office 2003 package. To characterize quantitative variables with a normal distribution, the arithmetic mean (M), standard deviation (SD), and 95% confidence interval were determined. To describe qualitative ordinal variables, the median (Me), upper and lower quartiles (UQ; LQ), maximum (Max) and minimum (Min) values were calculated. Qualitative nominal variables were characterized by relative and absolute frequencies (proportions). In the process of visual analysis of histograms and the Shapiro-Wilk method, the normality of the distribution of the studied quantitative variable was determined. Levene's test was used to evaluate the difference in variances.
Table 1. Comparative characteristics of the main demographic indicators of the study sample with the general population of MS patients in the Republic of Tatarstan
Parametric statistical methods (paired t test) were used to compare normally distributed quantitative variables in related and unrelated groups. Nonparametric statistical methods were used to compare qualitative ordinal variables: the Mann-Whitney test was used for intergroup comparisons, and the Wilcoxon test was used for intragroup comparisons before and after treatment. To compare groups on qualitative nominal variables, the z test, χ2 test, and Fisher’s exact test were used (in case chi-square was not applicable). Results were interpreted as statistically significant if the p
was less than 5% (
p
<0.05).
results
The average age of patients in the study sample was 27 [5; 24] years (from 18 to 37 years), male/female ratio (M/F) - 15/25 (37.5/62.5%), disease duration 30.08 [11; 36] months (from 12 to 63 months), EDSS in exacerbation - 3 [2.5;4.5] points (from 1.5 to 4.5 points).
When comparing the demographic indicators of the study sample in the general population of patients with MS in the Republic of Tatarstan [10], who are undergoing dispensary observation at the Republican CDC of the Department of Health of the Ministry of Health of the Republic of Tatarstan, the ratio of men/women is generally similar to the situation in the general population (15/25 and 475/1135; p
=0.358), at the same time, there was a shift in the age of patients in the study sample towards a younger age - 27.1±5.2 and 43.8±12.9 years, respectively (
p
<0.001).
The duration of the disease in the sample was shorter than in the population as a whole (30.1±11.4 and 169.1±103.1 months, respectively; p
<0.001). These differences are due to the peculiarities of the conditions for inclusion in the study, suggesting that patients have a relapsing-remitting course of MS with frequent relapses, which is most typical for the initial stages of the disease in young patients (Table 1).
Clinical and demographic characteristics of the groups are presented in Table. 2. Intergroup differences in the compared indicators did not reach statistical significance ( p
>0.05).
Clinically, exacerbation of MS in groups was categorized as follows: retrobulbar neuritis (15 and 30%, respectively; p
= 0.26), brainstem dysfunction (15 and 25%;
p
= 0.43), hemispheric dysfunction (50 and 35%;
p
= 0.34), cervical incomplete transverse myelitis (20 and 10%;
p
= 0.38) (Table 3). In the 1st group, 18 (90%) patients received treatment with DMT, in the 2nd group - 19 (95%) patients.
Table 2. Clinical and demographic characteristics of the examined patients
Table 3. Clinical characteristics of exacerbations of MS in both groups.
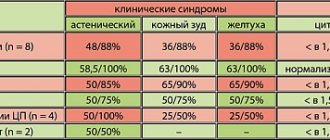
All patients in both groups showed certain neurophysiological abnormalities (Table 4). In group 1, changes in VEP were noted in 17 (85%) patients, TCMS - in 16 (80%), MR - in 10 (50%), SSEP - in 6 (30%), CRL/DLR - in 18 (90%). In the 2nd group, deviations from VEP were noted in 16 patients (80%), TCMS - in 14 (70%), MR - in 12 (60%), SSEP - in 8 (40%), CRL/DLR - in 17 (85%). There were no significant intergroup differences in deviations in NFO indicators.
Table 4. NFO indicators in both groups before treatment
The full course of treatment was completed by 17 (85%) patients in group 1 and 18 (90%) in group 2. The reasons for dropping out from the study were missed therapy (>2 infusions), which was interpreted as low adherence to treatment, affecting the subsequent analysis of the results.
Clinically, both groups showed regression of EDSS values (2.0[1.75;2.5] and 2.5[1.75;2.5], respectively), while there were no significant differences between groups ( p
=0.665) (Fig. 1).
In group 1, there was a statistically more pronounced improvement in performance by more than 5% in MSFC and SDMT testing ( p
= 0.038 and
p
= 0.026, respectively).
At the same time, no significant intergroup differences in the dynamics of improvement in VCAT values were identified ( p
= 0.658) (Table 5).
Table 5. Dynamics of indicators on the MSFC, SDMT, VCAT scales after treatment (points; improvement in indicators more than 5%)
Rice. 1. Score on the EDSS scale in groups immediately after pulse therapy with methylprednisolone and after completion of treatment with Cerebrolysin or placebo. In both groups, there was a statistically significant decrease in EDSS values, while at the same time, no significant intergroup differences were identified. The abscissa axis is the group; a — retrobulbar neuritis; b - stem form; c - hemispherical shape; g - myelitis; d - hemispheric-stem form.
Rice.
2. Comparative dynamics of the analyzed NFO indicators after treatment. The x-axis shows groups of patients. In group 1, there was a more significant regression of total deviations according to the results of the NFE than in group 2 (70.59 and 27.78%, respectively; p
=0.028).
When assessing the positive therapeutic effect with the preservation of residual total neurophysiological abnormalities in group 1, there is a tendency towards a more significant normalization of indicators than in group 2, which in turn did not reach statistical significance (64.7 and 27.78%, respectively; p
=0.064) (Table 6).
The least common in both groups was complete regression of pathological total deviations according to the NFO data - in the 1st group it was observed in 5.88%, while in the 2nd it was absent ( p
= 0.486).
Negative dynamics in NFO indicators during treatment occurred in 11.7% of patients in group 1, and in 33.3% in group 2 ( p
= 0.228).
Table 6. Dynamics of changes in NFO indicators before and after treatment
During the course of treatment in both groups of patients, a total of 5 non-severe adverse events (AEs) were observed (3 in the 1st group and 2 in the 2nd group; p
=0.66), not related to the use of the study drug and did not serve as a reason for its temporary or permanent withdrawal, as well as changes in the regimen of its administration (Table 7). There were no cases of non-severe AEs caused directly by treatment or serious AEs.
Table 7. Characteristics of non-severe AEs in both groups
According to MRI data, treatment was not accompanied by an increase in CO (the number of CO before treatment in 4 patients was 14, after treatment - 12), which argues against the potential inducing effect of exogenous AEs on the intrathecal inflammatory process in MS.