Instructions for use of the drug Polyoxidonium®, solution for injection and topical use
Azoximer bromide has a complex effect: immunomodulatory, detoxifying, antioxidant, moderate anti-inflammatory. The basis of the mechanism of immunomodulatory action of Azoximer bromide is a direct effect on phagocytic cells and natural killer cells, as well as stimulation of antibody formation and the synthesis of interferon-alpha and interferon-gamma. The detoxification and antioxidant properties of Azoximer bromide are largely determined by the structure and high-molecular nature of the drug. Azoximer bromide increases the body's resistance to local and generalized infections of bacterial, fungal and viral etiology. Restores immunity in secondary immunodeficiency conditions caused by various infections, injuries, complications after surgery, burns, autoimmune diseases, malignant neoplasms, the use of chemotherapeutic agents, cytostatics, steroid hormones. The use of the drug Polyoxidonium® against the background of secondary immunodeficiency states can increase the effectiveness and shorten the duration of treatment, significantly reduce the use of antibiotics, bronchodilators, glucocorticosteroids, and extend the period of remission. The inclusion of the drug Polyoxidonium® in the complex therapy of cancer patients reduces intoxication during chemotherapy and radiation therapy, in most cases allows for standard therapy without changing the regimen due to the development of infectious complications and side effects (myelosuppression, vomiting, diarrhea, cystitis, colitis and others ). A characteristic feature of Azoximer bromide when applied locally (intranasally, sublingually) is the ability to activate factors of the body's early defense against infection: the drug stimulates the bactericidal properties of neutrophils, macrophages, enhances their ability to absorb bacteria, increases the bactericidal properties of saliva and secretions of the mucous membranes of the upper respiratory tract. Azoximer bromide blocks soluble toxic substances and microparticles, has the ability to remove toxins and heavy metal salts from the body, and inhibits lipid peroxidation both by intercepting free radicals and by eliminating catalytically active Fe2+ ions. Azoximer bromide reduces the inflammatory response by normalizing the synthesis of pro- and anti-inflammatory cytokines. Azoximer bromide is well tolerated, does not have mitogenic, polyclonal activity, antigenic properties, does not have allergenic, mutagenic, embryotoxic, teratogenic and carcinogenic effects. Azoximer bromide is odorless and tasteless, and does not have a local irritant effect when applied to the mucous membranes of the nose and oropharynx.
Experience of clinical use of Polyoxidonium in complex therapy of respiratory diseases
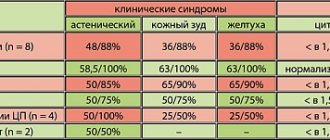
We chose the drug Polyoxidonium as an immunomodulatory agent. Its mechanism of action is based on direct activation of phagocytic cells and natural killer cells, increased interaction between T and B lymphocytes, and activation of antibody formation. At the same time, Polyoxidonium does not affect indicators that are within normal values, does not disrupt the natural mechanisms of inhibition of immune reactions, and does not deplete the reserve capabilities of the hematopoietic system. Polyoxidonium can be prescribed to patients with both identified and undiagnosed disorders of the immune status, based on clinical data. Along with immunomodulatory properties, Polyoxidonium also has detoxification and antioxidant properties, and also activates reparative and regenerative processes. An important feature of Polyoxidonium, which distinguishes it from other immunomodulators, is that it can be used both for the treatment of acute and chronic infectious processes of various etiologies, as well as for atopic diseases. These properties of the drug determined our choice of using it in patients with bronchopulmonary pathology. We used polyoxidonium in addition to complex therapy carried out in accordance with accepted standards of treatment. The study included 89 people. aged from 32 to 68 years, of which 60.7% were men, 39.3% were women. The study group of patients was represented by the following respiratory diseases: chronic obstructive pulmonary disease – 25 people. (28.1%), bronchial asthma – 38 people. (42.7%), bronchiectasis – 24 people. (27%), bronchopulmonary aspergillosis – 2 people. (2.2%). 22 people (35% of patients in the first two groups) fungal superinfection of the bronchi was detected. In 64% of cases, patients, in addition to bronchopulmonary diseases, had concomitant pathology, namely: duodenal ulcer, gastroesophageal reflux disease, intestinal dysbiosis, hypertension, myocardial dystrophy, fungal skin lesions. Methods of administration of Polyoxidonium were determined by the specific clinical situation. Patients received the drug intravenously, 6–12 mg, up to 5 injections per course (23 cases), intramuscularly, 6 mg up to 5–10 injections per course (13 cases), endobronchially, 6–12 mg, 1–2 procedures per course ( 53 cases). In 17 cases there was a combination of different methods of drug administration (intravenous and endobronchial). The endobronchial route of administration is possible due to the fact that Polyoxidonium is highly soluble in saline, quickly distributed in body tissues and does not cause local irritation. Endobronchial administration was carried out in the form of pouring a solution of the drug in a dose of 6 mg into the bronchi, as well as according to the well-known method of introducing 6 mg of Polyoxidonium, dissolved in 3 ml of saline solution into the carina of the trachea. For comparison, we took a group of patients comparable to the study group in terms of size, nosology, severity, age and gender. The dynamics of the patients' condition was assessed according to clinical indicators, data from laboratory, biochemical, radiological, bacteriological, endoscopic, and cytological research methods. The completeness of the therapeutic effect, the time of normalization of parameters, and the tolerability of treatment were compared in patients of the study and control groups. The assessment of the main clinical symptoms of exacerbation of the disease (cough, shortness of breath, volume and character of sputum, the presence of suffocation) was carried out in points (Table 1), by analogy with the criteria proposed by N. Anthonisen (1987). Patients in the study and control groups received standard therapy appropriate to the diagnosis and severity of the disease. The results of therapy were assessed according to the instrumental examination of patients, the severity of clinical symptoms (in points) and the rate of their regression (in days). Statistical data processing was carried out using variation statistics methods. As our observations showed, in the group of patients who received Polyoxidonium as part of complex therapy, there was a more rapid improvement in well-being, increased physical activity, and positive dynamics in the symptoms of the main and concomitant diseases. All patients tolerated the drug administration well. Only in three cases of endobronchial administration were short-term low-grade fever, tachycardia, and a transient increase in blood pressure noted, which we regarded as a general reaction of the body to the procedure; At the same time, bronchospasm was not recorded in any case of observation, with any method of drug administration. Tracking the clinical effects of Polyoxidonium in various nosologies, the following can be noted: the most clear result in our study was obtained when Polyoxidonium was prescribed to patients with exacerbation of bronchiectasis. The number of this group is 24 people, ages from 32 to 65 years, of which 66.7% are men, 33.3% are women. It should be noted that patients in the study and control groups had a long history of the disease, repeated exacerbations, requiring hospital treatment and the use of various antibiotics. Polyoxidonium in the study group of patients was prescribed at a dose of 6–12 mg in the form of intravenous infusions (5 procedures per course) in cases and (or) by endobronchial administration (1–2 procedures per course) in 20 cases; in 7 cases there was a combination of both methods of administration. Changes in the main clinical symptoms were assessed using a scoring system (according to Table 1) before the start of therapy, 1–2 days, 3–5 and 10–12 days from the start of treatment. The results of clinical observation are presented in Figure 1, where the columns of the chart reflect the severity of individual clinical symptoms (in points) in patients in the study group who received Polyoxidonium, and the graphs show the severity of the same symptoms (in points) in patients in the control group who did not receive this drug. As can be seen from the presented graphs and diagrams, patients in the study group experienced a more pronounced and rapid decrease in cough and “purulence” of sputum compared to the control group. The volume of sputum remains significant in the first days of treatment, and by days 10–12 it decreases significantly compared to the control group. Thus, in this case, the positive effects of Polyoxidonium include a faster and more pronounced reduction in the clinical symptoms of inflammation and improvement in bronchial drainage. This pattern was confirmed by the results of laboratory, bacteriological and endoscopic studies. The rapid detoxification effect was also noteworthy, literally from the first procedure of administering the drug. All this made it possible to reduce the dose of antibacterial drugs when carrying out the necessary sanitation of the bronchial tree. The indicated positive effects of Polyoxidonium with varying degrees of severity were observed in 100% of cases in the patients of the study group with bronchiectasis observed by us. The other study group consisted of patients with bronchial asthma (23 women, 15 men) aged from 38 to 60 years, all with polysensitization. The duration of the disease was 10 years or more. Moderate doses of systemic and inhaled glucocorticosteroids were used as basic anti-inflammatory therapy for 8 years or more. In addition, all patients in this group were characterized by a torpid course of the disease, with frequent exacerbations; the majority had side effects of glucocorticoid therapy (Cushingoid syndrome, osteoporosis). In 31.5% of cases, patients had mucosal and cutaneous candidiasis, requiring the addition of antifungal agents to therapy. Polyoxidonium was prescribed at a dose of 6 mg intravenously in 19 cases, intramuscularly in 6 cases, endobronchially (1-2 procedures per course) in 20 cases, in 7 cases there was a combination of different methods of administration. At the same time, no deterioration during the asthmatic process or manifestations of drug intolerance were recorded. In general, we observed a positive effect in 81.6% of cases. In 18.4% there were no significant clinical and functional changes. The dynamics of the main clinical symptoms before treatment and during observation were also assessed using a scoring system (Table 1). The results of clinical observation of the study group are summarized in a graph/diagram presented in Figure 2. Here, the columns of the chart reflect the indicators of the study group, and the graphs reflect the indicators of the control group (in points). As can be seen from Figure 2, with the introduction of Polyoxidonium, a more complete and rapid “effect of inflammation subsidence” is achieved, an increase in sputum production is observed due to improved bronchial drainage, and bronchial patency improves. More objectively, the improvement in bronchial obstruction during treatment is reflected by spirometry indicators (in particular, FEV1) in experimental bronchial asthma patients (who received Polyoxidonium) and control groups (Fig. 3). The rapid subsidence of inflammatory changes and a decrease in the phenomena of bronchial hyperactivity in patients of the study group led to the possibility of reducing the maintenance dose of glucocorticoids, reducing the need for b2-agonists without worsening the patients' condition. In the group of patients with chronic obstructive pulmonary disease who were prescribed Polyoxidonium, there were 25 people, of which 92% were men. This group is represented by patients with moderate and severe disease who have stage II respiratory failure. The disease occurred with frequent exacerbations requiring hospital treatment, with repeated courses of antibacterial, anti-inflammatory and constant bronchodilator therapy. In 40% of cases, the course of the disease was complicated by the addition of fungal superinfection of the bronchi (mucosal candidiasis). These patients, in combination with traditionally used medications, were prescribed Polyoxidonium in the form of intramuscular injections (7 cases), intravenous infusions (8 cases), and endoscopically (13 cases); in 3 cases there was a combined administration of the drug by different routes. To analyze the effectiveness of the drug in a scoring system (Table 1), the main clinical symptoms (cough, volume and character of sputum, shortness of breath and the number of asthma attacks) were assessed before the start and during treatment according to observation days: 1–2, 7–10, 12– 14th days. The results of clinical observations are summarized in graphs/diagrams (Fig. 4, 5). Assessing the results of the study (Fig. 4), it can be noted that when Polyoxidonium is prescribed in patients with COPD, the cough decreases faster and more clearly, literally on the 2-3rd day from the start of therapy the nature of the sputum changes from purulent, mucopurulent to mucous, and the volume of sputum even increases compared to the control group. Thus, the administration of Polyoxidonium leads to improved bronchial drainage, gives a more complete anti-inflammatory effect, leading to the possibility of sanitation of the bronchi without the use of antibiotics or with their short courses. As can be seen from Figure 5, symptoms reflecting respiratory disorders changed little over 12–14 days of observation. Patients in the study group noted an improvement in general well-being, an increase in resistance to habitual physical activity, but the objective functional indicators of spirometry practically remained the same (Fig. 6). In general, in the group of patients with chronic obstructive pulmonary disease, in 92% of observations, the effect of using Polyoxidonium was assessed as positive, of varying degrees of severity. Neither subjective nor objective deterioration in the condition of patients in this group was recorded when using Polyoxidonium. The dependence of the severity of the therapeutic effect of Polyoxidonium on the method of its administration into the body was studied (Table 2). The medicinal properties of the drug, namely anti-inflammatory effect, detoxification effect, enhancing the antimicrobial effect of antibacterial drugs, improving bronchial drainage, and the possibility of reducing the dose of other medications are assessed using a 5-point system: (–) – no effect. (+–) – questionable effect, (+) – significantly positive effect, (++) – significant effect, (+++) – maximally pronounced effect. The results are shown in Table 2. According to our observations, to achieve a clinically significant effect there is no need for long courses of use of the drug. Already with the first administrations of Polyoxidonium, changes in the clinical condition of patients are noted, and with the endobronchial method, 1–2 procedures are sufficient to obtain a significant result. With injection methods, 5–10 procedures per course are sufficient. No side effects were observed with all methods of administration, including in patients with atonic diseases. The speed of onset of the therapeutic effect, the multifunctionality of the effect on the state of the bronchopulmonary system and the body as a whole, the short duration of the course of administration, the absence of side effects when prescribing Polyoxidonium - all this significantly increases the effectiveness of standard therapy and reduces the patient’s time in the hospital (Fig. 7). Thus, the use of Polyoxidonium in the treatment of respiratory diseases is highly effective, expedient and allows optimizing anti-inflammatory treatment regimens for chronic nonspecific lung diseases.
Indications for use
Polyoxidonium has a wide list of indications and can be used as monotherapy for:
- prevention of influenza and acute respiratory viral diseases;
- prevention of complications after operations (bacterial infections).
The drug can also be used as part of complex therapy in the following cases:
- for infections of a viral or bacterial nature of the respiratory, reproductive and urinary systems;
- for allergic diseases that are complicated by the addition of bacteria, viruses or fungi;
- for rheumatoid arthritis;
- for tuberculosis;
- with intestinal dysbiosis;
- as well as for cancer tumors during and after chemotherapy, radiation.
Important!
Any use of this drug must be prescribed by a doctor. Self-medication with Polyoxidonium is unacceptable under any circumstances!