Home | About us | Delivery | Advertisers | Login | Registration
- Medicines
- dietary supplementsVitamins
- Categories from A to Z
- Brands from A to Z
- Products from A to Z
- Medical equipment
- beauty
- Child
- Care
- Honey products appointments
- Herbs and herbal teas
- Medical nutrition
- Journey
- Making medicinesStock
Pharmacy online is the best pharmacy in Almaty, delivering medicines to Almaty. An online pharmacy or online pharmacy provides the following types of services: delivery of medicines, medicines to your home. Online pharmacy Almaty or online pharmacy Almaty delivers medicines to your home, as well as home delivery of medicines in Almaty.
my basket
Apteka84.kz is an online pharmacy that offers its customers medicines, medicinal and decorative cosmetics, dietary supplements, vitamins, baby food, intimate products for adults, medical equipment and thousands of other medical and cosmetic products at low prices. All data presented on the Apteka84.kz website is for informational purposes only and is not a substitute for professional medical care. Apteka84.kz strongly recommends that you carefully read the instructions for use contained in each package of medicines and other products. If you currently have any symptoms of the disease, you should seek help from a doctor. You should always tell your doctor or pharmacist about all the medicines you take. If you feel you need further help, please consult your local pharmacist or contact our GP online or by telephone.
© 2021 Pharmacy 84.
Ketorolac
According to the World Health Organization (WHO), adverse events are classified according to their frequency as follows: very common (≥ 10%), common (≥ 1% and < 10%), uncommon (≥ 0.1% and < 1% ), rare (≥ 0.01% and < 0.1%), very rare (< 0.01%), frequency unknown (frequency cannot be determined from available data).
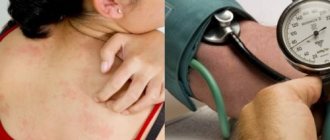
Allergic reactions
: uncommon - anaphylaxis or anaphylactoid reactions (change in facial skin color, skin rash, urticaria, skin itching, tachypnea or dyspnea, swelling of the eyelids, periorbital edema, shortness of breath, difficulty breathing, heaviness in the chest).
Local reactions:
common - burning or pain at the injection site.
From the central nervous system:
very common - headache; frequent - dizziness, drowsiness, increased sweating; uncommon - tremor, unusual dreams, hallucinations, euphoria, extrapyramidal symptoms, vertigo, paresthesia, depression, insomnia, nervousness, pathological thinking, loss of concentration, hyperkinesis, confusion (stupor), aseptic meningitis (fever, severe headache, convulsions, stiffness of the neck and/or back muscles), psychosis, fainting.
From the skin:
common - itching, rash (including maculopapular rash); uncommon - urticaria, toxic epidermal necrolysis (Lyell's syndrome), malignant exudative erythema (Stevens-Johnson syndrome), exfoliative dermatitis (fever with or without chills, flushing, thickening or peeling of the skin, enlargement and/or soreness of the tonsils).
From the urinary system:
uncommon - hematuria, proteinuria, urinary retention, oliguria, polyuria, frequent urination, acute renal failure, low back pain with or without hematuria and/or azotemia, interstitial nephritis, hyponatremia, hyperkalemia, hemolytic uremic syndrome (hemolytic anemia, renal failure, thrombocytopenia, purpura).
From the digestive system:
very common - gastralgia, dyspepsia, nausea; frequent - diarrhea, constipation, flatulence, feeling of fullness in the stomach, vomiting, stomatitis; uncommon - increased or decreased appetite, anorexia, erosive and ulcerative lesions of the gastrointestinal tract (including with perforation and/or bleeding - abdominal pain, spasm or burning in the epigastric region, blood in the stool or melena, vomiting with blood or coffee-ground type, nausea, heartburn), cholestatic jaundice, hepatitis, hepatomegaly. acute pancreatitis, polydipsia, dry mouth: frequency unknown - exacerbation of ulcerative colitis or Crohn's disease.
From the hematopoietic organs:
common - purpura; uncommon - anemia, eosinophilia.
From the respiratory system:
Uncommon: bronchospasm or shortness of breath, pulmonary edema, rhinitis, laryngeal edema (difficulty breathing).
From the senses:
Uncommon: taste disturbance, visual impairment (including blurred vision), hearing loss, ringing in the ears.
From the cardiovascular system:
frequent - increased blood pressure; infrequent - palpitations, pallor of the skin, fainting, hyperemia; frequency unknown - decreased blood pressure, heart failure, myocardial infarction, stroke.
From the hemostasis system:
uncommon - bleeding from a postoperative wound, nosebleeds, rectal bleeding.
Others
: frequent - swelling; uncommon - weight gain, fever, infections, asthenia, increased sweating, swelling of the tongue; frequency unknown - increased concentrations of urea and creatinine in the blood plasma.
Directions for use and doses
The drug must be administered intravenously over at least 15 seconds (for dosage forms containing ethanol).
Intramuscularly administered slowly, deep into the muscle, in minimally effective doses, selected in accordance with the intensity of pain and the patient’s response. If necessary, additional opioid analgesics can be prescribed at the same time in reduced doses.
Single doses for a single intramuscular or intravenous administration:
- patients 16-64 years old with a body weight of more than 50 kg - 10-30 mg, depending on the severity of the pain syndrome;
- patients 16-64 years old with body weight less than 50 kg, patients over 65 years old or with impaired renal function - 10-15 mg.
Doses for repeated parenteral administration:
Intramuscular:
-patients 16-64 years old with a body weight of more than 50 kg are given 10-30 mg for the first injection, then 10-30 mg every 4-6 hours;
- with a body weight of less than 50 kg, patients over 65 years of age or with impaired renal function - 10-15 mg every 4-6 hours.
Intravenously:
-patients 16-64 years old with a body weight of more than 50 kg are injected with 10-30 mg, then 10-30 mg every 6 hours, with continuous infusion using an infusion pump, the initial dose is 30 mg, and then the infusion rate is 5 mg /hour;
-patients 16-64 years old with body weight less than 50 kg, patients over 65 years old or with impaired renal function are administered a bolus of 10-15 mg every 6 hours.
The maximum daily dose for patients 16-64 years old with a body weight of more than 50 kg should not exceed 90 mg, and for patients 16-64 years old with a body weight less than 50 kg, over 65 years old or with impaired renal function - 60 mg as for intramuscular, and intravenous routes of administration.
Continuous intravenous infusion should not last more than 24 hours.
When administered parenterally, the duration of treatment should not exceed 2 days.
When switching from parenteral administration of the drug to oral administration, the total daily dose of both dosage forms on the day of transfer should not exceed 90 mg for patients 16-64 years old with a body weight of more than 50 kg and 60 mg for patients 16-64 years old with a body weight less than 50 kg, over 65 years of age or with impaired renal function. In this case, the dose of the drug in tablets on the day of transition should not exceed 30 mg.
Ketorolac solution for intramuscular administration 30 mg/ml in ampoules 1 ml No. 10
Name
Ketorolac solution for intramuscular injection 30 mg/ml in amp. 1 ml in pack No. 10
Description
transparent liquid of light yellow color with a faint odor.
Main active ingredient
ketorolac tromethamine
Release form
Solution
Dosage
30mg/ml
Indications for use
Acute severe pain syndrome in the postoperative period (as an alternative to opioids for the treatment of postoperative pain).
Directions for use and doses
Usual dosage regimen. The recommended single dose for adults is 10-30 mg. The maximum daily dose is 90 mg. The dosage should be adjusted depending on the intensity of the pain. The risk and severity of side effects can be reduced by selecting the lowest effective dose and short duration of use of the drug. The daily dose should be divided into several single doses, up to 4/day (every 6-8 hours). The maximum recommended dose should not be exceeded. Parenteral administration of the drug is intended for the treatment of acute and severe pain and should not be used for more than 2 days. The solution should be administered intramuscularly slowly over at least 15 seconds. If necessary, treatment can be continued with ketorolac in tablet form. A combination of two dosage forms is possible, taking into account the maximum daily dose of 90 mg.
Use during pregnancy and lactation
Forbidden.
Contraindications
Hypersensitivity to the active substance or any of the auxiliary components. History of bronchospasm, urticaria, or allergic symptoms after taking aspirin or other NSAIDs. Third trimester of pregnancy and during childbirth. Acute or history of gastric and/or duodenal ulcer, gastrointestinal bleeding or perforation. Inflammatory bowel diseases (eg, Crohn's disease, ulcerative colitis). Severe liver failure (liver cirrhosis and ascites). Moderate to severe renal impairment and hypovolemia or dehydration. Severe renal failure (creatinine clearance less than 30 ml/min). Severe heart failure (III-IV degrees). Treatment of postoperative pain after coronary artery bypass grafting (or use of a heart-lung machine). High risk of postoperative bleeding (for example, after tonsillectomy) or impaired hemostasis, hematopoiesis, cerebrovascular hemorrhage. Combined use with lithium preparations, pentoxifylline, probenecid or large doses of anticoagulants. Combined use with aspirin or other non-steroidal anti-inflammatory drugs. Ketorolac is contraindicated for prophylactic pain relief before surgery, as it inhibits platelet aggregation and increases the risk of bleeding during surgery. Neuraxial (epidural or intrathecal) administration of the injection solution due to alcohol content. Children under 16 years of age (safety and effectiveness have not been established).
Compound
1 ml of solution contains: active ingredient - ketorolac tromethamine - 30 mg; excipients: sodium chloride, disodium edetate, rectified ethyl alcohol, tromethamine solution 0.5 M, water for injection.
Pharmacokinetics
Absorption when administered intramuscularly is complete and rapid. After intramuscular administration of 30 mg of the drug, the maximum concentration in blood plasma (Cmax) is 1.74-3.1 μg/ml; 60 mg – 3.23-5.77 µg/ml, time to reach maximum concentration, respectively – 15-73 minutes and 30-60 minutes; the maximum concentration after intravenous infusion of 15 mg is 1.96-2.98 mcg/ml, 30 mg is 3.69-5.61 mcg/ml. The time to reach equilibrium concentration (Css) with parenteral administration is 24 hours when administered 4 times a day (above the subtherapeutic dose) and with intramuscular administration is 15 mg - 0.65-1.13 mcg/ml, 30 mg - 1.29- 2.47 mcg/ml, with intravenous infusion of 15 mg – 0.79-1.39 mcg/ml, 30 mg – 1.68-2.76 mcg/ml.
99.2% of the drug binds to blood plasma proteins and with hypoalbuminemia the amount of free substance in the blood increases.
The volume of distribution is 0.15-0.33 l/kg. In patients with renal failure, the volume of distribution of the drug may increase by 2 times, and the volume of distribution of its R-enantiomer by 20%. Poorly passes through the blood-brain barrier and penetrates the placenta (10%). Found in small quantities in breast milk.
More than 50% of the administered dose is metabolized in the liver with the formation of pharmacologically inactive metabolites. The main metabolites are glucuronides, which are excreted by the kidneys, and p-hydroxyketorolac, which is excreted 91% by the kidneys, 6% through the intestines.
The half-life (T1/2) in patients with normal renal function averages 5.3 hours (3.5-9.2 hours after intramuscular administration of 30 mg and 4-7.9 hours after intravenous administration of 30 mg). The half-life is prolonged in elderly patients and shortened in young patients. Liver function does not affect the half-life. In patients with impaired renal function with a plasma creatinine concentration of 19-50 mg/l (168-442 µmol/l), the half-life is 10.3-10.8 hours, with more severe renal failure - more than 13.6 hours.
The total clearance is with intramuscular administration of 30 mg - 0.023 l/kg/h (0.019 l/kg/h in elderly patients), intravenous administration of 30 mg - 0.03 l/kg/h; in patients with renal failure with a plasma creatinine concentration of 19-50 mg/l with intramuscular administration of 30 mg - 0.015 l/kg/h.
Not excreted during hemodialysis.
There is information about the effectiveness of the drug for migraine attacks.
Interaction with other drugs
The simultaneous use of ketorolac with acetylsalicylic acid or other NSAIDs, including cyclooxygenase-2 inhibitors, calcium preparations, glucocorticosteroids, ethanol, corticotropin can lead to the formation of gastrointestinal ulcers and the development of gastrointestinal bleeding.
The drug should not be used simultaneously with other NSAIDs (including cyclooxygenase-2 inhibitors), as well as simultaneously with probenecid, pentoxifylline, acetylsalicylic acid, lithium salts, anticoagulants (including warfarin and heparin).
Do not use with paracetamol for more than 2 days. Co-administration with paracetamol increases nephrotoxicity, and with methotrexate - hepato- and nephrotoxicity. Co-administration of ketorolac and methotrexate is possible only when using low doses of the latter (monitor the concentration of methotrexate in the blood plasma).
Probenecid reduces the plasma clearance and volume of distribution of ketorolac, increases its concentration in the blood plasma and increases its half-life. With the use of ketorolac, the clearance of methotrexate and lithium may decrease and the toxicity of these substances may increase.
Co-administration with indirect coagulants (for example, warfarin), heparin, thrombolytics, antiplatelet agents, cefoperazone, cefotetan and pentoxifylline increases the risk of bleeding.
Reduces the effect of antihypertensive and diuretic drugs (the synthesis of prostaglandins in the kidneys is reduced). When combined with narcotic analgesics, the doses of the latter can be significantly reduced.
Antacids do not affect the complete absorption of the drug.
The hypoglycemic effect of insulin and oral hypoglycemic drugs increases (dose recalculation is necessary).
Co-administration with valproic acid causes disruption of platelet aggregation. Increases the plasma concentration of verapamil and nifedipine.
When prescribed with other nephrotoxic drugs (including gold preparations), the risk of developing nephrotoxicity increases.
Drugs that block tubular secretion reduce the clearance of ketorolac and increase its concentration in the blood plasma.
It is necessary to take into account possible interactions when using ketorolac simultaneously with cyclosporine, zidovudine, digoxin, tacrolimus, quinoline drugs, selective serotonin reuptake inhibitors, and mifepristone.