Relevance
The benefit of using dexamethasone or betamethasone in pregnant women at risk of preterm birth has been shown in countries with high health care costs.
However, the cost-effectiveness of this intervention in countries with low health care costs is unknown. A new study assessed the effectiveness and safety of antenatal glucocorticoid use in women in countries with low health care costs.
Dexamethasone solution for injection 4 mg/ml ampoules of 1 ml 25 pcs.
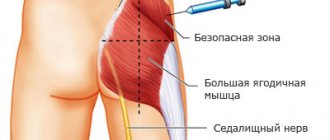
Doses are set individually for each patient, depending on the nature of the disease, the expected duration of treatment, tolerability of glucocorticosteroids and the patient's response to the therapy. The injection solution can be administered intravenously (in the form of injections or infusions with glucose solution or saline), intramuscularly and locally (intra-articularly, into skin lesions, into soft tissues). Parenteral use. Parenteral dexamethasone is administered in acute cases when oral therapy is not possible and for the conditions described in the “Indications for Use” section. The recommended average initial daily dose for IV or IM use ranges from 0.5 mg to 9 mg and, if necessary, up to 24 mg (equivalent to 1/3-1/2 oral dose). The initial dose of dexamethasone should be continued until clinical response is achieved; then the dose is gradually reduced to the minimum effective. If high doses are used for more than a few days, the dose of the drug should be gradually reduced over the next few days or longer. Long-term treatment should be carried out at a dose not exceeding 0.5 mg/day. Local application. The recommended single dose of dexamethasone for intra-articular administration is from 0.4 mg to 4 mg. The dose depends on the size of the affected joint. The usual dose of dexamethasone is 2 mg to 4 mg for large joints and 0.8 mg to 1 mg for small joints. Intra-articular administration can be repeated after 3-4 months. More frequent administration of dexamethasone can lead to damage to intra-articular cartilage and bone necrosis. The usual dose of dexamethasone for intrabursa administration is 2 mg to 3 mg, for tendon sheath administration is 0.4 mg to 1 mg, and for tendon sheath administration is 1 mg to 2 mg. When administered into limited lesions, the same doses of dexamethasone are used as for intra-articular administration. The drug can be administered simultaneously into a maximum of two lesions. The recommended dose of dexamethasone for soft tissue inflammation (periarticular administration) is 2 mg to 6 mg. Use in children. When administered intramuscularly, the dose for replacement therapy is 0.02 mg/kg body weight or 0.67 mg/m2 body surface area, which is divided into 3 injections with an interval of 2 days, or from 0.008 mg to 0.01 mg/m2. kg body weight or 0.2 mg to 0.3 mg/m2 body surface area daily. For other indications, the recommended dose is 0.02 mg to 0.1 mg/kg body weight or 0.8 mg to 5 mg/m2 body surface area, every 12 to 24 hours. Emergency situations. The initial dose is 4-20 mg, which is repeated until the desired effect is achieved, the total daily dose rarely exceeds 80 mg. After achieving a therapeutic effect, dexamethasone is administered at a dose of 2-4 mg as needed, followed by gradual withdrawal of the drug. To maintain a long-term effect, the drug is administered every 3-4 hours or as a long-term drip infusion. After relief of acute conditions, the patient is transferred to oral dexamethasone. In case of shock, administer strictly intravenously as a bolus at a dose of 2-6 mg/kg. If necessary, repeated doses are administered every 2-6 hours or as a long-term intravenous infusion at a dose of 3 mg/kg/day. Treatment with dexamethasone should be carried out as part of complex therapy for shock. The use of pharmacological doses is permissible only in life-threatening conditions, and, as a rule, this time does not exceed 48-72 hours. For cerebral edema, an initial dose of 10 mg is administered intravenously, then 4 mg every 6 hours until symptoms resolve (usually in within 12-24 hours). After 2-4 days, the dose is reduced and the use of the drug is gradually stopped over 5-7 days. Patients with malignant neoplasms may require maintenance treatment - 2 mg IM or IV 2-3 times a day. In case of acute cerebral edema, short-term intensive therapy is carried out: the loading dose for adults is 50 mg IV, then on days 1-3 8 mg is administered every 2 hours, on the 4th day - 4 mg every 2 hours, on days 5- Day 8 - 4 mg every 4 hours, then the daily dose is reduced by 4 mg/day until it is completely discontinued. For children weighing more than 35 kg, the loading dose is 25 mg IV, then 4 mg every 2 hours on days 1-3, 4 mg every 4 hours on days 4, 4 mg every 4 hours on days 5-8, 4 mg every 6 hours, then the daily dose is reduced by 2 mg/day until it is completely discontinued. For children weighing less than 35 kg, the loading dose is 20 mg IV, then 4 mg every 3 hours on days 1-3, 4 mg every 6 hours on days 4, 2 mg every 6 hours on days 5-8. mg every 6 hours, then the daily dose is reduced by 1 mg/day until the drug is completely discontinued. For acute self-limiting allergic reactions or exacerbation of chronic allergic diseases, parenteral and oral administration of dexamethasone is combined: day 1 - iv 4-8 mg, day 2-3 - orally 1 mg 2 times a day, day 4-5 - orally 0 .5 mg 2 times a day, 6-7 days - 0.5 mg orally once. On the 8th day, the effectiveness of therapy is assessed.
results
- The study included 2852 women and their 3070 fetuses. The women were observed in hospitals in Bangladesh, India, Kenya, Nigeria and Pakistan.
- The study was completed earlier than planned because the second interim analysis showed a clear benefit from steroid therapy.
- Neonatal death occurred in 278 of 1417 infants (19.6%) in the dexamethasone group and in 331 of 1406 infants (23.5%) in the placebo group (relative risk, 0.84; 95% CI, 0.72-0. 97; P=0.03).
- Stillbirth or neonatal death occurred in 393 of 1532 fetuses and infants (25.7%) in the dexamethasone group and in 444 of 1519 fetuses and infants (29.2%) in the placebo group (relative risk, 0.88; 95% CI, 0.78-0.99; P=0.04).
- The incidence of maternal bacterial infection was 4.8% with dexamethasone compared with 6.3% in the control group (relative risk, 0.76; 95% CI, 0.56-1.03).
- According to the safety analysis, there were no significant differences between the groups in the frequency of side effects.
Dexamethasone
special instructions
Caution should be used in case of parasitic and infectious diseases of a viral, fungal or bacterial nature (currently or recently suffered, including recent contact with a patient) - herpes simplex, herpes zoster (viremic phase), chicken pox, measles, amoebiasis, strongyloidiasis (established or suspected), systemic mycosis; active and latent tuberculosis. Use for severe infectious diseases is permissible only against the background of specific therapy.
It should be used with caution within 8 weeks before and 2 weeks after vaccination, with lymphadenitis after BCG vaccination, with immunodeficiency conditions (including AIDS or HIV infection).
Use with caution in diseases of the gastrointestinal tract: gastric and duodenal ulcers, esophagitis, gastritis, acute or latent peptic ulcers, recently created intestinal anastomosis, nonspecific ulcerative colitis with the threat of perforation or abscess formation, diverticulitis.
Should be used with caution for diseases of the cardiovascular system, incl. after a recent myocardial infarction (in patients with acute and subacute myocardial infarction, the necrotic focus may spread, slowing down the formation of scar tissue and, as a result, rupture of the heart muscle), with decompensated chronic heart failure, arterial hypertension, hyperlipidemia), with endocrine diseases - diabetes mellitus ( including impaired tolerance to carbohydrates), thyrotoxicosis, hypothyroidism, Itsenko-Cushing's disease, with severe chronic renal and/or liver failure, nephrourolithiasis, with hypoalbuminemia and conditions predisposing to its occurrence, with systemic osteoporosis, myasthenia gravis, acute psychosis , obesity (III-IV degree), with poliomyelitis (with the exception of the form of bulbar encephalitis), open- and closed-angle glaucoma.
If intra-articular administration is necessary, it should be used with caution in patients with a general severe condition, ineffectiveness (or short duration) of the action of 2 previous administrations (taking into account the individual properties of the GCS used).
Before and during GCS therapy, it is necessary to monitor a general blood count, glycemic levels and plasma electrolyte levels.
For intercurrent infections, septic conditions and tuberculosis, simultaneous antibiotic therapy is necessary.
Relative adrenal insufficiency caused by dexamethasone may persist for several months after its discontinuation. Taking this into account, in stressful situations that arise during this period, hormonal therapy is resumed with the simultaneous administration of salts and/or mineralocorticoids.
When using dexamethasone in patients with corneal herpes, the possibility of corneal perforation should be kept in mind. During treatment, it is necessary to monitor intraocular pressure and the condition of the cornea.
With the sudden withdrawal of dexamethasone, especially in the case of previous use in high doses, a so-called withdrawal syndrome occurs (not caused by hypocortisolism), manifested by anorexia, nausea, lethargy, generalized musculoskeletal pain, and general weakness. After discontinuation of dexamethasone, relative adrenal insufficiency may persist for several months. If stressful situations arise during this period, GCS is prescribed (according to indications), if necessary in combination with mineralocorticoids.
During the treatment period, monitoring of blood pressure, water and electrolyte balance, peripheral blood picture and glycemic level, as well as observation by an ophthalmologist is required.
In children, during long-term treatment, careful monitoring of the dynamics of growth and development is necessary. Children who during the treatment period were in contact with patients with measles or chickenpox are prescribed specific immunoglobulins prophylactically.
Dexamethasone reduced heavy periods in clinical trials
Wikimedia Commons
British doctors conducted clinical trials of the use of dexamethasone to treat heavy periods. A dosage of the drug of 1.8 milligrams per day reduced the volume of menstrual flow in participants by 25 milliliters. Now scientists have to monitor how well the drug is tolerated over time. The results of the study were published in EBioMedcicine
.
Heavy menstruation is a period that lasts more than 7 days or requires changing hygiene products more than once every two hours. The problem of heavy menstrual bleeding affects one out of three women. This significantly reduces quality of life and can also lead to anemia. Most often, hormonal drugs are used to treat heavy menstruation - birth control pills, which are not suitable for women planning a pregnancy.
Scientists led by Hilary Octavia Dawn Critchley from the University of Edinburgh conducted a series of laboratory experiments in which they found that heavy bleeding from the uterus is due to a lack of glucocorticoid hormones in the endometrium. They suggested that such women could be helped by giving them dexamethasone during the luteal phase of the cycle, when endometrial vessels are differentiating. They recruited women into the clinical trial whose menstrual blood loss was equal to or greater than 50 milliliters across two screening menstrual cycles. The volume of blood released during menstruation was measured from the hygiene products used using the alkaline-hematine method. A total of 107 women participated in the study: 27 were placed in the placebo group and 80 in the dexamethasone group. The drug was given once a day for five days of the luteal phase at a dosage of 0.4 milligrams to 1.8 milligrams. The study lasted five months.
In clinical studies, the most effective dosage of the drug was 1.8 milligrams: in women taking it, the volume of menstrual blood decreased by 25 milliliters (<0.05). Adverse events (not necessarily related to treatment) were reported in 75 percent of women receiving dexamethasone and 58 percent of participants receiving placebo.
Effect of different dosages of dexamethasone
Warner et al/EBioMedicine, 2021
Share
Clinical trials have shown promise for dexamethasone in treating heavy periods, but more research will be needed before this treatment is approved. In them, scientists will have to include more participants and monitor the tolerability of dexamethasone over a longer period of time. Researchers are also thinking about creating a dosage form of the drug that would deliver dexamethasone locally to the uterus.
Previously, scientists have developed feminine hygiene products that detect vaginal candidiasis. The presence of fungus is indicated by the pink coloration of the gasket.
Anastasia Kuznetsova-Fantoni