Design of DNA vaccines
To obtain DNA vaccines, the gene encoding the production of an immunogenic protein must be inserted into a vector, the role of which is played by a bacterial plasmid or virus [4]. The vector should not replicate in the cells of the macroorganism, therefore it can only contain prokaryotic replication initiation sites.
To create DNA vaccines, well-studied plasmids of Gram-negative bacteria (mainly E. coli), in particular multicopy pUC19 or pBR322 and their derivatives, are used. Special vectors for DNA vaccines have been developed—pcDNA3 and pcDNA3.1 (Invitrogen), which contain a cytomegalovirus (CMV) promoter and a polyadenylation signal for the bovine growth hormone gene. Other commercially available plasmids that are most commonly used as DNA vaccine vectors include: pVAX1 (Invitrogen), pCI, VR1012 DNA, pJW4303, pVAC1-mcs, and pVAC2-mcs (InvivoGen). The last two are used to enhance the humoral immune response and contain antigens to the surface structures of muscle cells [5].
Among the viral vectors that provide a higher level of expression of the target antigen, the most commonly used are: replication-defective adenovirus serotype 5 (AD5), orthopoxviruses and modified vaccinia viruses, and alphaviruses. The adenoviral vector has a high transfection efficiency - up to 100%, and can include up to 8 kb. DNA. The negative point is the synthesis of its own proteins that can induce an immune response. The most used vaccinia modifications are Ankara (MVA) and New York Vaccinia strain (NYVAC). The first was obtained as a result of 56-fold passage of the virus in chicken embryonic fibroblasts. The NYVAC genome has deleted 18 open reading frames associated with host range and virulence. Up to 50 kb can be inserted into each of the listed vectors. DNA [6].
Innovative vaccine against COVID-19 will be available by autumn
Elena Kovacich, August 2, 2021, 19:45 — REGNUM “Betuvax-CoV-2” is the first subunit recombinant vaccine in Russia. This means that during its creation, only an artificially synthesized specific fragment of the coronavirus was used, which is key to the formation of an immune response.”
Vaccine
Ivan Shilov © IA REGNUM
The vaccine, fundamentally different from other Russian vaccines against COVID-19, was developed by the Human Stem Cell Institute (HSCI). “Betuvax-Kov-2” was released by HSCI under the patronage, so to speak, of the Platform of the National Technology Initiative (PNTI). The application from PNTI has already been submitted to the Ministry of Health of the Russian Federation.
“Betuvax-Kov-2,” the TASS announcement says, “is a subunit recombinant vaccine.” Since most of us, although more experienced in terms of the coronavirus topic, are still not doctors or virologists, I decided to turn to the developers. I would like to clarify the details and ask questions about the new product that is being prepared for production after testing and issuance of permission by the Ministry of Health. So far, as TASS reports, “based on the results of preclinical studies, the vaccine has shown safety and effectiveness in animal models.” Now it is important to understand how a person will react to a new product.
With a request for an interview, I turned to the press secretary of the administration of PJSC “Human Stem Cell Institute” (HSCI) Elena Romanova . quite young, existing since 2020. Naturally, I was curious how they managed to release the innovative drug “Betuvax-CoV-2” so quickly, they themselves have only existed for a year?!
According to the HSCI representative, despite such a short period of time—only a year—the developed product “showed a good safety and efficacy profile with the formation of high titers of neutralizing antibodies against the SARS-CoV-2 coronavirus in animal models. The developers were faced with the task of creating a vaccine that would potentially have a minimum of unwanted effects that may be associated with vaccination. This safety profile will make the vaccine in demand for people with various chronic diseases, older age groups and, possibly, children.”
What is the difference between Betuvax-CoV-2 and other vaccines?
HSCI: “The Betuvax-CoV-2 vaccine is the first new generation recombinant vaccine in Russia based on spherical particles made from natural material that imitate viral particles containing the surface antigen (protein) of the coronavirus. This vaccine design activates the production of only the necessary protective antibodies and cellular immunity, while reducing the theoretical risks of antibody-dependent enhancement of infection. The design of the Betuvax-CoV-2 vaccine also makes it possible to quickly optimize it for new strains of coronavirus and reuse it multiple times. This vaccine does not contain genetic material, excess antigenic load, additional preservatives, stabilizers and inorganic adjuvants, which minimizes the risks of side effects.”
Pasteur observes guinea pigs. Book illustration
Is Betuvax-CoV-2 better protected against COVID-19? If yes, why?
HSCI: Betuvax-CoV-2 is the first subunit recombinant vaccine in Russia. This means that during its creation, only an artificially synthesized specific fragment of the coronavirus was used, which is key to the formation of an immune response. The vaccine does not contain the whole virus (like the killed virus vaccine CoviVac) or other viruses used as a delivery vector (like Sputnik V). That is, a vaccine based on technology such as Betuvax-CoV-2 will be the first in Russia. Subunit vaccines are recognized as the safest on the market, which is why, for example, the usual flu vaccine is most common in the recombinant subunit version format. This type of vaccine is the least reactogenic; no viral material is used in their production, so subunit vaccines do not lead to the development of acute inflammatory side effects, like other types of vaccines - vector or based on whole viruses.”
Cellular technologies raise concerns and controversy in terms of the possible provocation of cancer. How safe is the vaccine for people in this sense?
HSCI: “Preclinical studies on animals have shown the safety of the Betuvax-CoV-2 vaccine in the studied doses and the absence of visible side effects. Clinical trials are needed to prove the complete absence of side effects in humans.”
How soon will Betuvax be widely used?
HSCI: Betuvax has submitted a dossier to the Ministry of Health to obtain permission to conduct clinical trials of the vaccine. Now the department is reviewing the application and, according to the standard procedure, asking the developers additional questions.”
Cells
Joseph Elsbernd
On my own behalf, I will also add as an explanation that experts distinguish three main approaches to the development of vaccines depending on what is used for immunization: 1) whole virus or bacteria, 2) fragments of the virus that cause an immune response, 3) only genetic material containing code for the synthesis of specific proteins, rather than a whole virus. So Betuvax, as a subunit vaccine, uses fragments of the virus that our immune system must become familiar with. These fragments do not contain whole microorganisms or safe viruses as a vector. Which, in fact, is what we have in the case of CoviVac and Sputnik. So this is something different, and hopefully even more effective. Although the vaccine is called an innovation, its type is the same as most older vaccines - whooping cough, tetanus, diphtheria and meningitis.
In a word, while someone is looking for people who are immune to Covid or is preparing to leave in the event of the Apocalypse to New Zealand, where it seems safe in terms of Covid, Russian scientists are almost churning out vaccine after vaccine. Therefore, there is a chance that the Apocalypse will not come. After all, those five countries, quite small, which are named the safest in the event of a fatal outcome of the pandemic - New Zealand, Iceland, Tasmania, Ireland and the UK - will not be able to accommodate all the hungry. Therefore, it is better to look for a way to survive at home. Besides, we are not Silicon Valley billionaires: they are the ones buying up plots and houses in New Zealand. Most Russians, due to the lack of billions, cannot get into the coveted top five. We must save ourselves here, at home, and now...
Constructor elements
Figure 3. Design of a DNA vaccine based on the pVAX1 vector with a chimeric gene (Rat cDNA, Human cDNA). Pcmv—cytomegalovirus promoter; MCS—multiple gene cloning site; BGH pA - terminator with a signal for polyadenylation of the bovine growth hormone gene; Kanamycin—kanamycin resistance gene; pUC ori—the origin of replication of pUC group plasmids; HindIII, BstEII, XbaI - restriction sites. Figure from [5].
To be useful for creating DNA vaccines, every self-respecting vector must contain the necessary structural elements (Fig. 3).
- Structures ensuring plasmid replication (ori pUC19, pMB1 are used) and restriction sites.
- Selective markers: genes for resistance to antibiotics (but not to penicillin and other β-lactam antibiotics) [5].
- CpG motifs of bacteria, which, due to the lack of methylation, are able to enhance the immune response. This principle underlies the development of a universal vaccine and implies the use not of genes encoding microbial antigen proteins, but of bacterial CG sequences as the active component of the vaccine [7].
- The Kozak sequence is a consensus sequence surrounding the start codon (GCC(A/G)CC AUG G) that plays an important role in translation initiation in eukaryotes.
- A promoter for the expression of a target gene in eukaryotic cells. The most commonly used promoters are the SV40 virus, cytomegalovirus (often together with intron A), the beta-actin promoter, promoters specific to certain tissue types (for example, the desmin gene promoter for expression in myocytes, the vitamin D3 hydroxylase gene promoter in keratinocytes, albumin - in hepatocytes). The use of the promoter and synthesis system of bacteriophage T7 allows expression of the target gene without the participation of the transcriptional system of the cells of the macroorganism and, accordingly, without moving the vector into the nucleus [8].
- Target gene encoding a pathogen protein. It may also contain additional nucleotide sequences encoding ligands for receptors on antigen presenting cells. Such sequences can be the genes of the CD40 marker protein, the extracellular domain of Fms-like tyrosine kinase-3, or T-killer antigen-4. Facilitating antigen degradation in the proteasome or lysosome will also stimulate the immune response. Therefore, to enhance the proteolytic cleavage of the antigen, a ubiquitination signal is inserted into its sequence [9, 10].
- The target gene is followed by polyadenylation signals, for example from the SV40 virus, the rabbit β-globin gene or bovine growth hormone.
- Stop codons close this chain, and double or triple termination sequences (TAGTGATGA) are often used.
Delivery services
The methods of introducing DNA vaccines into the body are given no less attention than the creation of the constructs themselves, since the success of immunization as a whole depends on this. Therefore, various, sometimes very ingenious, methods of delivering such vaccines into the body have been developed.
Figure 4. PowderJect's disposable gene gun. a - appearance; b - in section. Drawing from the website www.apteka.ua.
The simplest is the parenteral route of administration , which involves injecting DNA vaccines in saline solution (intramuscular, intradermal). In this case, most of the DNA enters the intercellular space and only then is incorporated into the cells.
Using the gene gun . To do this, DNA is fixed on microscopic gold beads (about 1–2 microns), and then, using a device driven by compressed helium, the beads are “shot” directly into the cells (Fig. 4). This delivery method requires significantly less injected material than intramuscular injection. So, for injection into mice, 10-100 mcg of the drug is needed, and using a gene gun, 0.1-1 mcg is enough.
Electroporation is a technique that uses electrical impulses to form pores in the cell membrane and deliver DNA directly into cells.
Microcontainers made of polymaterials . Moscow scientists, for example, have created a porous microsphere of calcium carbonate, coated with several layers of polysaccharides, into which a DNA molecule is packaged. If microspheres in a polymer shell are placed in an acidified solution, the calcium carbonate inside will dissolve and leave through the polymer membrane. Only the DNA to be transported will remain inside. Not many similar microcontainers have been developed for DNA delivery. There are foreign analogues in which the capsule shell is made of polylactic acid. They are used to create vaccines against hepatitis and even AIDS. The average diameter of microcapsules for delivering DNA vaccines is only 1–2 microns. Such microcapsules can be administered subcutaneously or even into the blood. If an enzyme is placed into a microparticle along with DNA or a drug, which breaks down the capsule shell from the inside, then the release of the drug can be controlled: the less enzyme, the slower the shell collapses.
Liposomal carriers provide high delivery efficiency when administered intravenously, while the expression of target genes increases significantly, as it occurs in many organs, and especially in the spleen.
DNA vaccines can be administered orally using bacterial carriers . For these purposes, for example, modified bacteria Shigella flexneri with a deletion in the asd gene are used. Mutant bacteria grow in vitro on a medium with diaminopimelic acid and, penetrating into eukaryotic cells, do not reproduce in them, since the mentioned acid is absent, but produce antigens encoded in the plasmid [6]. For oral administration, a vector was created based on a weakened Salmonella strain, which is capable of self-destruction in the body after a certain period of time after completing immunization tasks. To do this, the bacterium was modified in such a way that its survival became dependent on the presence of artificial sugars not found in the body. After cells infected with a genetically engineered Salmonella strain run out of the specific sugar supplied with the vaccine, the bacteria are unable to maintain the integrity of their cell walls, which leads to their death [11].
An original system for DNA delivery was proposed using “shadows” - non-living cells of gram-negative bacteria, devoid of cytoplasmic contents, but retaining morphology and antigenic structures, including adhesive factors. “Shadows” have tropism for antigen-presenting cells of the macroorganism and adjuvant properties that enhance the immune response. In addition, in a freeze-dried state, “shadow” preparations are stored at room temperature indefinitely, and their production is cheap [6].
A technology for delivering DNA vaccines using bacteriophages [12]. In this case, vaccine DNA is inserted into the genome of a bacteriophage vector, which is then used to immunize the macroorganism [13].
It must be taken into account that different methods of delivering DNA vaccines into the body ensure the development of different cellular reactions, while important immunological pathways can be stimulated or, conversely, not involved during the development of a protective response. Methods and sites of administration of DNA vaccines vary among different species of organisms. For example, the ears of a pig are an excellent place for injection, but injecting the drug into the ears of sheep or cows is ineffective.
Instructions for use of the Sputnik V vaccine: mechanism of action and how the vaccination should take place
We tell you the history of its creation, the mechanism of action, the full name of the Sputnik V vaccine against coronavirus from the Gamaleya Center, what the instructions for using the drug say, as well as how the vaccination should take place.
Sputnik V is the first coronavirus vaccine registered in Russia. Its registration name is “Gam-COVID-VAK” (lat. Gam-COVID-Vak). The drug was developed at the NICEM named after. N.F. Gamaleya.
History of the vaccine
The name “Sputnik V” was not chosen by chance. According to the drug's website, it was named after the first Soviet space satellite. And the letter V (not the Roman five, as many are mistaken) comes from the word victory - “victory”.
During the development of the drug, the Gamaleya Center used a platform on which a number of other vaccines were created, including against Ebola. It is noted that the platform itself was developed about 25 years ago. It was planned to be used for gene therapy.
The first volunteers received the vaccine on March 30, 2021. Then only 19 days had passed since the WHO declared a coronavirus pandemic. Three weeks later, the volunteers received a second injection. As of August 2021, all trial participants had good immunity. There were no serious side effects recorded.
How the vaccine works
The drug consists of two solutions that contain recombinant human adenoviruses Ad5 and Ad26. They are used as a “transport” (or vector) to deliver the gene that encodes the S protein of the coronavirus SARC-CoV-2.
Two injections are needed for a reason: during the first vaccination, the vector enters the cell, and the body begins to synthesize the S-protein of the coronavirus. This causes a response - the development of immunity. After 21 days, re-vaccination occurs with another vector, which further “spurs up” the immune response, allowing the formation of longer-term immunity against infection.
How should the vaccination take place?
The vaccine is administered intramuscularly to people over 18 years of age. Before the injection, the doctor must measure blood pressure, body temperature and check blood saturation. Contraindications to the drug include hypersensitivity to its components, severe allergic reactions, exacerbation of infectious and non-infectious diseases, pregnancy and breastfeeding.
In case of exacerbation of chronic infectious and non-infectious diseases, the drug is administered 2-4 weeks after recovery or remission.
After vaccination, redness and discomfort may appear at the injection site. However, in most cases, this goes away quickly. In addition, fever may be a side effect of the vaccine. There is no need to worry in this situation either. You should consult a doctor if the temperature persists for several days.
Dear readers of Constantinople!
If you have something to share with the editorial office of Tsargrad St. Petersburg, send your observations, questions and news to our VKontakte page or by email
And also join us in Yandex.Zen.
Genetic vaccine helpers
To enhance the immune response caused by DNA vaccines, various adjuvants are administered together with them, for example, plasmids encoding the synthesis of cytokines, granulocyte-macrophage colony-stimulating factor and other costimulatory molecules (B7.1 (CD80), B7.2 (CD86) and CD40 ) [14].
A design has been created for the DNA vaccine against HIV that ensures a higher titer of antibodies and its persistence for a longer time compared to a conventional DNA vaccine. This molecular virus-like construct is particles with a diameter of 25-30 nm containing a polynucleotide complex in the center - the recombinant plasmid pGEX-2T-TBI with the genes of the infectious agent HIV-1 or double-stranded RNA, which is a stimulator of nonspecific resistance of the body. On the surface of the construct are hybrid proteins containing HIV-1 epitopes and an enzyme (for example, glutathione-S-transferase or galactosidase). The connection between the polynucleotide complex and hybrid proteins is carried out through a conjugate: spermidine (for connecting the conjugate with the polynucleotide complex) - polyglucin - a substrate for the enzyme (for example, glutathione or galactopyranoside; for affinity sorption of hybrid proteins onto the construct).
Current state of DNA vaccinology
Currently, about 420 DNA vaccines are in development against diseases of various etiologies in both humans and animals.
The majority of anti-infective therapeutic DNA vaccines being developed target HIV-1. Significant advances have been made in active immunization against human papillomavirus. Some vaccines are at the clinical trial stage and may soon be introduced into mandatory practice. Thus, the American company Inovio, specializing in the development of DNA vaccines, has created a drug against cervical dysplasia VGX-3100, which is undergoing the second phase of clinical trials. In 2013, VGX-3100 received the "Best Therapeutic Vaccine" award at the World Vaccine Congress. The following are in phase I or IIa clinical trials: vaccines against hepatitis C, cervical cancer, head and neck cancer, AIDS, and influenza. Inovio is also actively developing vaccines against Ebola* and prostate cancer.
* - For a more familiar, but no less promising method of combating the Ebola virus - using a “cocktail” of monoclonal antibodies - read the article “Ebola virus and rhesus monkeys: a new effective medicine has been obtained” [15]. - Ed.
Other organizations are also paying great attention to the development of methods for vaccine therapy of oncological diseases using recombinant DNA. The DNA vaccine against leukemia, created at the University of Southampton (but administered using an electroporator from the same Inovio), showed good effectiveness. The vaccine is aimed at suppressing the activity of the WT1 (Wilms tumor gene) gene in the body. It is the increased activity of this gene that is observed in tumor cells of various types. During phase I clinical trials, patients were observed to develop an immune response, including the activation of killer T cells and the production of antibodies; The safety of the new vaccine has also been proven. The trials have entered phase II, but due to funding problems, the organizers are not yet able to increase the number of participants [16].
Animals need the same protection as people. In this regard, DNA vaccines are being developed for veterinary medicine against bovine and equine herpes viruses, canine distemper virus, classical swine fever virus, rabbit papilloma, foot and mouth disease, infectious hematopoietic necrosis virus, influenza virus, Japanese encephalitis virus, rabies virus, vesicular stomatitis virus, etc. .d. [13]. Many DNA vaccines are being developed to combat viral, bacterial and eukaryotic fish pathogens [17].
DNA vaccines are being actively developed to improve the immunity of birds. Multicomponent DNA vaccines can reduce the number of vaccinations required during the short lifespan of birds and avoid the risk of increased virulence of some pathogens. In the case of poultry farming, the problem is that vaccines are introduced into the amniotic fluid of eggs, which has DNase activity, so the properties of the DNA vaccine may be impaired. Encapsulating DNA in cationic liposomes will likely help solve this problem.
Of the many DNA vaccines developed, only a few have been licensed to date, and only animals are lucky in this regard (Table 1).
Table 1. Licensed DNA vaccines [18].
| Trade name of the vaccine | Year of licensing | Target | Animal | Vaccine product | The purpose of creating a vaccine |
| West Nile-Innovator (USA) | 2005 | West Nile virus | Horses | Structural protein of the PreM-E virus | Protection against the virus |
| Apex-IH-N (Canada) Apex-IHN | 2005 | Causative agent of infectious necrosis of hematopoietic tissue (INGT) | Fish of the salmon family | Viral glycoprotein | Increasing the quantity and quality of fish products |
| LifeTide Es-Dable 5 (Australia) LifeTide® SW 5 | 2008 | A growth hormone | Pigs and other livestock | Porcine somatoliberin | Increasing litter size in sows; significantly reduces perinatal mortality and morbidity |
| "Oncept" (USA) | 2006 | Melanoma | Dogs | Human tyrosinase | As an alternative to radiation therapy and surgery in the treatment of melanoma |
| PsaA DNA vaccine (Brazil) | 2001 | Pneumonia | Studied in mice | Surface adhesin A of pneumococcus | Fighting Streptococcus pneumoniae |
| West Nile Virus DNA Vaccine (USA) USDA: 1995.D0 | West Nile virus | Sei whale (saidyan or willow whale) | Structural protein of the virus | Virus protection |
These vaccines, approved by the US Food and Drug Administration (FDA), are based on plasmids. For the first three of them, the method of administration recommended by the manufacturer is intramuscular; for the LifeTide vaccine, it is intramuscular injection combined with electroporation. If other vaccines are aimed at activating the immune system, then for the LifeTide drug the immunostimulating effect is additional. The vaccine product, somatoliberin, stimulates the pituitary gland to release growth hormone and prolactin. Their effect in pigs leads to an increase in the weight of animals and an increase in the number of litters. At the same time, the introduction of a plasmid that encodes somatoliberin stimulates the production of T-lymphocytes and natural killer cells, therefore increasing the body's immune resistance.
The procedure for introducing drugs into veterinary practice is simpler than into human clinical practice, which is why some DNA vaccines are already used to immunize animals.
Vaccines are routinely used to immunize foxes orally against rabies. Recombinant vaccines V-RG (Vaccinia Rabies Glycoprotein - smallpox glycoprotein of the rabies virus) are created by introducing into the genome of the smallpox virus a DNA sequence encoding the glycoprotein of the rabies virus. Vaccination of foxes began in 2007, and by 2009 the incidence had decreased by 85% [19].
Antitumor drugs have been created that have demonstrated high effectiveness in animals and are used in veterinary practice. For the treatment of melanoma in dogs, the DNA vaccine Oncept has been used in US veterinary medicine since 2007 (Fig. 5). Practice has shown that this drug cures melanoma or significantly prolongs the life of sick dogs [20].
Figure 5. DNA vaccine preparation against canine melanoma. Figure from [20].
The newer drug Elenagen was developed by an international consortium led by CureLab Oncology (USA). Although Elenagen is a new drug, extensive studies have already been carried out on various tumors in mice and rats. These studies using hundreds of animals showed that the drug inhibited the growth of sarcoma, breast cancer, lung carcinoma and melanoma, i.e. fast-growing and very aggressive tumors. Elenagen did not demonstrate any toxic side effects. Moreover, the drug was also effective in the case of canine lymphosarcoma. Currently, a study of the spectrum of antitumor action of Elenagen in dogs and cats has begun. Unlike Oncept, which is effective only against melanoma, the Elenagen vaccine suppresses the growth of both melanoma and other malignant tumors. Due to a more diverse mechanism of action, the new drug can have an additional healing and strengthening effect on the animal’s body.
* * *
Thus, with the help of DNA vaccines, it is possible to form stable immunity against infectious agents of various natures. They have been developed for more than 20 years in various laboratories around the world, but despite this, such vaccines are practically not used in clinical practice. This is due to the fact that there are still no answers to a number of questions related primarily to the safety of DNA vaccines. Although the available data do not give reason for special concern: these drugs are no more dangerous than many factors to which a person deliberately exposes himself on a daily basis. DNA vaccines may well be a miraculous discovery that will save many lives.
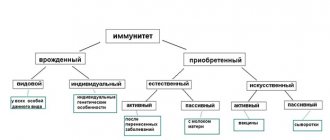
Types of vaccines
Vaccines contain active ingredients, or immunogens, and excipients. Immunogens are responsible for activating the immune system. Excipients are used to create vaccines with optimal quality composition, to increase their effectiveness, and increase shelf life.
There are different types of vaccines.
Live vaccines
Live vaccines are produced from living microorganisms with reduced virulence.
Most of these vaccines promote the development of long-term, high-level immunity. Live vaccines are against influenza, measles, mumps, yellow fever, etc. Inactivated (killed) vaccines
Inactivated (killed) vaccines are obtained by completely neutralizing bacteria and viruses while maintaining their immunogenic properties. There are whole cell, subunit, recombinant and split vaccines.
Whole cell (whole virion) vaccines
Whole-cell (whole virion) vaccines are prepared by freeze-drying (at low temperatures under vacuum), heating or treatment with chemicals (formalin, formaldehyde). These include vaccines against whooping cough (DPT), influenza, viral hepatitis A, tick-borne encephalitis, cholera, etc.
Subunit vaccines
Subunit vaccines contain only surface antigens, which reduces the protein content of the vaccine and, therefore, reduces its allergenicity. Subunit vaccines include vaccines against influenza, pneumococcal, meningococcal, hemophilus influenzae infections, etc. Split vaccines Split vaccines are made from destroyed viruses. They contain fragmented and purified particles, including surface proteins and other components of viruses. This group includes vaccines against influenza and others.
Recombinant vaccines
Recombinant vaccines are a new generation of immune drugs produced by inserting a viral antigen into the genome of yeast cells. A representative of this group is the vaccine against viral hepatitis B.
Anatoxins
Toxoids are made from exotoxins (toxins secreted by pathogens).
They are easily dosed and combined with other vaccines. When toxoids are administered, antitoxic immunity is developed. Diphtheria, tetanus, staphylococcal toxoids, as well as toxoids against botulism and gas gangrene are used. There are monovaccines
(containing one antigen), associated or combined (having several antigens), and polyvalent vaccines (consisting of different strains of the same type of microorganisms).
Excipients Vaccine excipients include adsorbents, preservatives, emulsifiers, pH indicators, and stabilizers. Adsorbents
(adjuvants) are insoluble aluminum salts (phosphate or hydroxide) that enhance the effect of the vaccine and, therefore, significantly increase the strength of the immune response.
Sometimes transport proteins are used as adsorbents (they are part of diphtheria and tetanus toxoids). Preservatives are needed to suppress the proliferation of “foreign” microorganisms. For this purpose, thiomersal (merthiolate), formaldehyde, phenoxyethanol, phenol and antibiotics (neomycin, gentamicin, polymyxin) are used. The content of preservatives in vaccines is extremely low, and in such concentrations they do not pose any danger. Small amounts of emulsifiers are added to improve the dissolution of dry vaccines. In the production of many dry vaccines, dextran, sucrose, sorbitol, gelatin, and albumin are used as stabilizers. Methyl red is often used as a pH indicator. You can immediately detect a “shift” in the acidity indicator by changing the color of the drug and reject the vaccine. Source: www.microgen.ru
News posted by the site:
Literature
- Vaccines in questions and answers;
- Wikipedia: “DNA vaccine”;
- Zverev V.V. (2006). Vaccine prevention: past, present, future. Bulletin of Biotechnology. 2, 58–62;
- Molecular cloning, or how to insert foreign genetic material into a cell;
- Montgomery DL, Prather KJ (2006). Design of plasmid DNA constructs for vaccines. Methods Mol. Med. 127, 11–22;
- Popov Yu.A., Mikshis N.I. (2010). Genetic (DNA) vaccines. Problems of especially dangerous infections. 105, 20–24;
- Olishevsky S.V., Kozak V.V., Yanish Yu.V., Rybalko S.L., Shlyakhovenko V.A. (2006). Immunostimulating CpG DNA: prospects for clinical use in oncology. Oncology. 8, 209–217;
- Donnelly JJ, Ulmer JB (1999). DNA vaccines for viral diseases. Braz. J. Med. Biol. Res. 32, 215–222;
- Starodubova E.S., Isagulyants M.G., Karpov V.L. (2010). Regulation of immunogen processing: signal sequences and their use to create a new generation of DNA vaccines. Acta Naturae. 2, 59–65.;
- Ubiquitin is ubiquitous;
- Kong W., Brovolda M., Koeneman B. A., Clark-Curtiss J., Curtiss III R. (2012). Turning self-destructing Salmonella into a universal DNA vaccine delivery platform. PNAS. 47, 19414–19419;
- Platform viruses: poison for good;
- Mateen I., Irshad S. (2011). A review on DNA vaccines. J. Health Sci. 1, 1–7;
- "Junk" DNA controls the evolution of mammals?;
- Ebola virus and rhesus macaque: a new effective drug has been obtained;
- DNA vaccine for leukemia. Inovio Pharmaceuticals website;
- Kurath G. (2008). Biotechnology and DNA vaccines for aquatic animals. Rev. Sci. Tech. 27, 175–196;
- DNAVaxDB: DNA Vaccine (electronic database of DNA vaccines);
- Gorbacheva P., Makarov V.V. (2010). Recombinant rabies vaccine for oral immunization of foxes. Veterinary pathology. 2, 16–18;
- ONCEPT® Web Site (Oncept drug site, Merial Ltd)..