Immunization protects pregnant women from vaccine-preventable diseases, followed by the transfer of acquired antibodies to the newborn. Antibodies passively acquired from the mother can protect the child from diseases in the first months of life. Maternal immunization is an important challenge facing public health practice.
Vaccination and vaccinations during pregnancy lead to the transplacental transfer of antibodies, which can provide protection to the newborn in the first months of life [17].
Widespread implementation of immunization of pregnant women, as an important public health goal for the prevention of neonatal diseases, in the United States and other industrialized countries faces obstacles stemming from theoretical doubts by expectant mothers about the safety of vaccines, relatively low awareness, and practical socioeconomic problems. The most important issue regarding immunization during pregnancy remains the safety of vaccines for the fetus and newborn [1]. The decision to vaccinate a pregnant woman is quite a responsible one. To make this decision, the doctor and the pregnant woman must jointly determine the potential risk of infection with a particular pathogen, as well as the possible consequences of infection for the mother and child. These risks must be weighed against the likely benefits of vaccination [2, 3].
Are live antiviral vaccines contraindicated during pregnancy?
It was believed that pregnancy is a contraindication to vaccination with live viral vaccines due to the theoretical risk of virus penetration through the placenta and intrauterine infection of the fetus. Live virus vaccines include attenuated influenza vaccine (LAIV), MMR (measles, mumps, rubella), and varicella, smallpox, and tuberculosis (BCG) vaccines. Current guidelines suggest that caution should be exercised when administering live viral vaccines during pregnancy. The US Advisory Council on Immunization Practices (ACIP) of the Center for Disease Control (CDC) does not consider vaccination to be an indication for termination of pregnancy [4, 5].
From 1979 to 1980 The CDC closely monitored the effects of RA 27/3 vaccine (live rubella virus vaccine) given to women within three months before or after conception; There are no data on the effect of the vaccine on the development of birth defects or congenital rubella syndrome [6]. Live attenuated polio vaccine was also well tolerated by pregnant women [7, 8].
When live viral vaccines (eg, rubella, chickenpox, OPV) were administered to pregnant women, there was no increase in the incidence of adverse events in the fetus or newborn. Some of these vaccines (eg, OPV, yellow fever) are indicated for pregnant women who are at high risk of exposure to infection.
The only live antiviral vaccine that is toxic to the fetus remains the smallpox vaccine. These vaccines should not be used in women of childbearing age without appropriate toasts confirming the absence of pregnancy, and should not be administered to family members of a pregnant woman.
Does a child need vaccinations?
Although rubella is not a fatal disease, there are factors that confirm the need to vaccinate your child. These include:
- If a child is not vaccinated before the age of six, there is a chance that he may develop progressive rubella panencephalitis. The outcome of this disease is fatal.
- In the case of a three-component vaccine, the child will receive protection not only from rubella, but also from measles and mumps, and therefore will not become infected with them, and will not suffer possible complications from diseases.
- Rubella causes serious complications if infected in adulthood. In order to prevent this, it is better to get vaccinated in childhood.
Hepatitis B
Compared with adults, newborns and infants infected with hepatitis B virus have a greater risk of developing severe liver damage and even death or chronic liver damage.
The hepatitis B vaccine includes hepatitis B surface antigen (HbsAg) particles. which are unable to cause infection. CDC believes that its use cannot cause harm to the fetus [10]. In contrast, almost 90% of children born to mothers infected with hepatitis B virus in the last trimester developed chronic hepatitis if they were not vaccinated at the time of birth [11].
Rubella vaccination - contraindications and complications
The vaccine is not given if the patient:
- serious allergy to the components of the drug;
- oncology;
- viral or chronic diseases in the acute stage;
- HIV.
The rubella vaccine is not given to pregnant women. After vaccination the following are possible:
- slight fever, weakness;
- joint pain;
- enlarged lymph nodes under the armpit or in the neck;
- red or purple rash.
Symptoms go away after a few days.
Tetanus
Tetanus toxoid administration is indicated for pregnant and lactating women who have not previously received immunization or require a booster injection. No negative effects of the tetanus vaccine on the fetus have been identified [12]. As a precaution, the CDC recommends vaccination after the end of the first trimester of pregnancy [5]. Although Tdap vaccination (acellular DTP vaccine with a reduced content of difluoride toxoid and pertussis component) during pregnancy is not specifically specified, there are also no contraindications to its administration.
Rubella vaccination for children and adults: features of the procedure
The vaccine must be stored and transported correctly. This is the only way it will remain effective. Therefore, choose serious clinics with a good reputation. If you don’t know where to get vaccinated against rubella, contact SANMEDEXPERT. The medical center is located in Moscow, in the Central Administrative District, near the Baumanskaya metro station. Here, the patient will be examined by a doctor before vaccination and, if necessary, will prescribe tests and a comprehensive examination. We offer:
- An inexpensive but effective mono-vaccine of domestic production.
- Complex vaccination against measles, rubella and mumps "Priorix", made in Belgium.
The first vaccination is carried out at 1 year. Usually they use the complex “triple” vaccination “Priorix” or its analogues. Repeated vaccination at 6 years of age. If the child cannot be vaccinated during the year, this can be done later. The interval between primary and re-vaccination is 6 years. A single vaccination protects for 6-11 years. After two injections, immunity lasts up to 20 years. Girls are recommended to repeat the vaccination at 12–14 years of age to eliminate risks during pregnancy. The procedure does not require special preparation:
- You need to be examined by a doctor and talk about allergies and recent illnesses.
- For several days before vaccination, you should, if possible, limit contact with other people. This way you will reduce the risk of contracting the flu or acute respiratory infections.
- Allergy sufferers are advised to take antihistamines 2-3 days before the procedure.
Rubella
When infected with the rubella virus in the first 4 weeks. During pregnancy, in approximately 50% of cases, the fetus develops congenital rubella syndrome (CRS). including blindness, heart defects, deafness and other developmental defects [13]. By the third trimester, the risk of developing CRS in the fetus decreases to 10%.
Rubella vaccine is a live antiviral vaccine. It should not be administered to pregnant women due to the theoretical possibility of exposure of the fetus to the live virus. However, there were no cases of CRS development in newborns whose mothers were unscheduledly vaccinated or became pregnant within 3 months. After vaccination All women of childbearing age should be screened for rubella antibodies. Most cases of CRS have been reported in newborns whose mothers were not screened or vaccinated despite medical supervision [14].
Contraindications
Only a doctor can decide whether Rubella is suitable for vaccination
The Rubella vaccine is contraindicated if there is a history of an allergic reaction to any component of the vaccine, as well as in the following cases:
- Allergic reactions to vaccine components.
- Acute infectious and non-infectious diseases, exacerbation of chronic diseases.
- Immunodeficiency conditions; malignant blood diseases and neoplasms.
- Pregnancy and breastfeeding period.
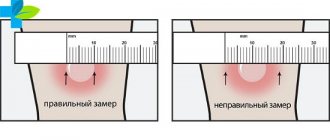
- A severe reaction (temperature rise above 40 C, swelling, hyperemia more than 8 cm in diameter at the site of vaccine administration) or a complication to the previous dose of the vaccine.
Chicken pox
Infection of a pregnant woman can lead to the development of congenital chickenpox syndrome in the fetus, characterized by limb atrophy, strictures, microcephaly, cortical atrophy, and chorioretinitis. cataracts and other defects [15]. Compared to the first trimester, the risk of developing congenital varicella syndrome is slightly higher in the period from 13 to 20 weeks. pregnancy [16]. The effect of the vaccine on the fetus has not been proven. The risk of developing congenital varicella syndrome after infection with wild type virus is 0.4-2.0%; When using an attenuated vaccine, the risk of developing congenital varicella syndrome should theoretically be lower or completely absent [16].
Complexes with this research
Non-child immunity Diagnosis of rubella, measles, chickenpox and mumps viruses RUB 2,950 Composition
Examination during pregnancy. 1st trimester 16,690 RUR Composition
Immunity strength Diagnosis of rubella, measles and mumps viruses RUB 2,140 Composition
IN OTHER COMPLEXES
- TORCH infections. Avidity of IgG antibodies 5,920 R
- Miscarriage RUB 40,070
- IVF planning RUB 12,990
- Pregnancy planning. Diagnosis of infections RUB 8,620
- Entry into IVF RUB 23,020
Hepatitis A
The safety of hepatitis A vaccination during pregnancy has not been studied; however, since the hepatitis A vaccine is based on inactivated hepatitis A virus, the possibility of infection is theoretically very small. The risk associated with vaccination must be weighed against the possible risk of developing hepatitis A in a pregnant woman, especially if the risk of exposure to infection is high [18 ].
Literature:
- Munoz FM, Englund JA, Infect. Dis. Clin. North Amor. 2001: 15(1): 253-271.
- Silvers MJ. Steploe MM. Prim. Care Clin. Off. Pracl. 2001:28: 1-9.
- AnmncanCollegeotObntotnciansandGynecoiogisls, Committee on Obstetric Practice. Immunization during pregnancy. ACOG Com Opin. 2003; 282.
- Centers for Disease Control arxl Prevention. General recommendations on immunization, recommendations of tl»e Advisory Committee on Immunization Practices (ACIP) and the American Academy of Family Physicians (AAFP) MMWR Morb. Mortal. Wkly Rep. 2002; 51 (RR02): 1-36.
- Centers for Disease Control and Prevention. Guidelines for vaccinating pregnant women. 2004. Available at: https://www.in.gov/iHdh/programs/immunization/lmmunzationSchedGuldelines%20for%20Vaccinating%20Pregnant%20Women.pdf. Accessed January 25.2008.
- American Academy of Pediatrics: Poliowus infections. In Pickering LK (ed): 2000 Red Book Report of the Committee on Infectious Diseases, ed 25. Elk Grove Village. IL. American Academy of Pediatrics. 2000. pp. 465 470.
- DaSilva MM, Prom KA. Johnson FA. et at. JAMA 1958:1681 -1685-
- Linder N. Hands!ler R. Fruman O. et al Pedi.itr Infect. Ois J. 1994; 13:959-962
- Naleway AL. Smith WJ, Mullooly JP. Epidemiol Rev. 2006; 28: 47-53.
- Centers for Disease Control and Prevention Connecticut. 1994-1995 and United States. 1979-1994. MMWR Morb Mortal. Wkly Rep. 1996: 45: 584 -587.
- Estaban R. Vaccine 1995; 13(suppl 1): S35 - S36.
- Silveira CM. Caceres VM, Dutra MG. Lopes-Camelo J. CastMla EE Bulletin WHO. 1995:73.605-608
- AmencanColk*geofKtetriciansandGynecologists. ACOG N(ws Release November 29, 2002.
- HacktoyBKJ Nurse Midwifery 1999.44:106-117. 15- Centers for Disease Control and Prevention. MMWR Morb. Modal. Wkly Rep. 1996: 45 (RR* 11): 1-25.
- Pastuszak AL. Levy M. Schick In el al N. Engl. J Med 1994; 330: 901 905
- Englund. J. et al. Path 2007: 137: S16 - S19 18 COC. MMWR 2006: 55 (No. RR-7): 15
- CDC. MMWR 2006; 55 (No. RR-7): 15.
Table 1. ACIP recommendations regarding immunization during pregnancy or lactation
| Vaccine/toxoid | Use during pregnancy | Use during breastfeeding | Comments |
| Live attenuated viral vaccines | |||
| measles | No | Yes | usually included in MMR |
| parotitis | No | Yes | usually included in MMR |
| rubella | No | Yes | usually as part of MMR, 4 weeks in advance. before planned conception |
| polio | not routinely used | not routinely used | can be used when traveling to endemic areas |
| yellow fever | No | No | if contact with infection is unavoidable, it is possible to use |
| chicken pox | No | Yes | planning pregnancy after 4 weeks. after vaccination |
| Killed or inactivated vaccines | |||
| flu | Yes | Yes | Vaccination should be offered to all pregnant women in the second and third trimester. Can be used in women at risk in the first trimester |
| rabies | Yes | Yes | the disease is fatal in almost 100% of cases |
| Hepatitis B | Yes | Yes | |
| Pneumococcal infection | Yes | Yes | recommended for women at risk |
| typhoid fever | No | No | only when traveling to endemic areas |
| anthrax | No | No | recommended only for women at risk |
| tetanus | Yes | Yes | |
| diphtheria | Yes | Yes | |
Flu shot
Influenza is dangerous for pregnant women; it can lead to serious complications and defects in the fetus, even leading to premature birth. Therefore, doctors recommend that even pregnant women be vaccinated during an epidemic. It is important to understand that the flu vaccine does not provide a 100% guarantee against infection. However, the disease will be easier.
Parents who want to ensure good health for their unborn child should start planning ahead not only for pregnancy, but also for conception. To do this, you need to register with a gynecologist, get tested in a timely manner, and get vaccinated in advance.
THIS IS NOT AN ADVERTISING. THE MATERIAL WAS PREPARED WITH THE PARTICIPATION OF EXPERTS.
Should you get vaccinated or not: pros and cons
Women have different attitudes towards vaccinations. Some consider vaccination to be the only right decision to protect themselves and their unborn child from fatal pathologies. Others are wary of getting immunized because of the potential for side effects and complications.
Doctors insist on preventing rubella before pregnancy. To understand whether it is worth getting vaccinated, you need to consider and weigh all the pros and cons.
Arguments in favor of rubella vaccination before conception:
- vaccination forms stable and long-lasting immunity;
- The rubella virus is easily transmitted and can cause fetal death and the birth of a child with defects. More than 50% of children are born with health conditions incompatible with life. prevention allows you to minimize disastrous consequences;
- vaccine effectiveness reaches 100%;
- immunization is well tolerated by most women;
- availability of a choice of vaccines;
- availability of prevention;
- Under 25 years of age you can get vaccinated for free.
Arguments against immunization:
- the need to renew protective forces through revaccination;
- slow antibody production;
- the presence of a number of contraindications;
- after vaccination you cannot become pregnant for three months;
- there is a possibility of complications developing.
There are more arguments for vaccination against rubella in preparation for pregnancy, and they are more significant. Thanks to prevention, it was possible to minimize the incidence of disease. Today, cases of rubella pathology are rare.
Recommendations for immunization should be made by the doctor individually in each specific case, based on the vaccination history, examination results, characteristics of the body and the patient’s health status.
Before you start planning for a baby, experts recommend taking a test for the concentration of antibodies in the blood.