Vaxigrip or Influvac, which is better? This question arises for everyone who decides to delve into the topic of immunization and choose the safest and most effective vaccine for themselves and their loved ones. Both drugs are modern developments of pharmaceutical companies, so they are purified to the maximum and contain only current influenza strains. It is preferable to make the final decision on the choice of vaccine together with a specialist who has sufficient knowledge to evaluate each of the presented vaccines.
Description
Immunization with Influvac vaccine prevents diseases caused by influenza A and B viruses and related strains. This vaccine does not contain live viruses. It is adapted to the mutations of influenza viruses that occur annually.
Children aged 6-36 months are given this vaccine in a dosage of 0.25 ml. Children over 3 years of age, adolescents and adults are given 0.5 ml of Influvac to prevent influenza. The seroprotective level of antibodies required to prevent the development of the disease is achieved within 10 days after vaccination. In this case, immunity lasts for 6-12 months. If a person has already been infected with the influenza virus when the vaccine is administered, the drug does not provide protection against the development of this disease.
Description of the drug Vaxigrip
In terms of release form, Influvac and Vaxigrip are no different - in both cases it is a suspension that can be injected subcutaneously or into muscle tissue. However, Vaxigrip is also available in ampoules.
If we consider the composition, then some differences are only in the list of excipients. If in the first case sodium phosphate dihydrate was present, then sodium chloride is used here. The same applies to potassium. Otherwise, the composition is no different.
The dosage of Vaxigrip is also no different from Influvac. It is worth noting that this drug is also given to people with immune problems. They need to administer 0.25 twice, with a break of a month.
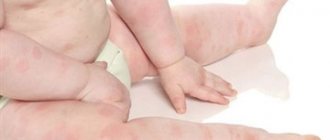
The possible side effects are also the same. For example, vaccinated people may:
- Get a headache
- Malaise develops
- Increased sweating
- Pain in muscles or joints appears,
- Neuralgia, paresthesia and encephalomyelitis begin,
- Reduce the amount of strength
- Allergic reactions, lumps at the injection site and vasculitis may develop.
So, it is obvious that there are no fundamental differences between Influvac and Vaxigripp. Their composition differs minimally, the dosages and list of side effects are almost the same. So how do you choose the best drug?
Special conditions
Influvac does not prevent upper respiratory tract diseases that are caused by viruses other than the influenza virus. This vaccine should be administered deep under the skin or intramuscularly. Under no circumstances should this drug be administered intravascularly. Therefore, the injection must be done with extreme caution to avoid the drug entering the vascular bed. The injection should warm to room temperature before administration. After vaccination, a person must remain under medical supervision for 30 minutes.
Directions for use and dosage
It is advisable to start using Influvac before the onset of autumn cold weather. The vaccine is prescribed by a pediatrician or therapist after a thorough examination to exclude inflammatory processes in the patient’s body.
The drug is administered once in a dose:
- 0.25 ml – children from 6 months to 3 years;
- 0.5 ml – for children over 3 years old and adults.
For previously unvaccinated children, it is recommended to repeat Influvac after a month.
The procedure for administering the drug should be carried out in a medical institution.
Drug interactions
The effectiveness of this vaccine may be reduced during immunosuppressive therapy and if the vaccine recipient has an immunodeficiency. This medicine should not be mixed with other medicines as its compatibility with other medicines has not been studied.
Immunization with Influvac can be done together with other vaccines, but injections must be given in different parts of the body. It should be borne in mind that the side effects of each of the vaccines administered to a person may be more acute.
special instructions
The treatment room must be provided with the mandatory availability of medications necessary to provide emergency care for anaphylactic shock.
The use of Influvac is indicated from the second trimester of pregnancy, and for women at risk - regardless of the gestation period.
The drug can be used during breastfeeding.
Patients should be informed that if enzyme immunoassay tests are performed after recent vaccination, it is possible to obtain false-positive results from serological tests when determining antibodies against HIV, human T-cell lymphotropic virus, and hepatitis C.
According to the instructions, intravenous administration of Influvac is strictly prohibited.
If it is necessary to simultaneously administer several vaccines, the procedures are carried out in different parts of the body and with separate syringes.
Indications
The indication for use of this vaccine is the prevention of influenza. Vaccination with this drug is recommended before the start of the epidemic influenza season. This vaccine can be used to immunize children from 6 months of age. Annual prevention of influenza using this vaccine is especially recommended for people who are in the following risk groups:
- age over 65 years;
- age from 6 months to 18 years;
- patients with chronic diseases of the respiratory system (including bronchial asthma), chronic renal failure, metabolic disorders (including diabetes mellitus);
- patients receiving radiation therapy, taking cytostatics or corticosteroids;
- patients with immunodeficiency conditions caused by diseases or medications;
- medical workers;
- schoolchildren and students;
- family members of persons at risk for influenza;
- children and adolescents who undergo long-term treatment with drugs containing acetylsalicylic acid.
Is it worth getting the flu vaccination?
The flu vaccine has the ability to form immunity against this dangerous virus. Vaccination is not a mandatory procedure, but WHO specialists recommend that certain categories of the population who have a particularly high risk of disease and complications undergo this procedure.
- Elderly people whose age is over 60-65 years.
- People (adults and children) with chronic diseases of the respiratory, cardiovascular, endocrine, urinary systems (diabetes mellitus, renal failure, cirrhosis and others).
- Patients of different ages with severe immunosuppression (presence of HIV, after undergoing radiation or chemotherapy).
- Workers in areas where they have to constantly be among a large number of people (doctors, teachers, military personnel, drivers, police officers, educators, salespeople).
- People living in closed institutions (correctional institutions, nursing homes, dormitories).
- Pregnant women, after the onset of the second trimester of gestation.
- Children attending preschool, school and other educational institutions.
We recommend reading: Drugs for the prevention of influenza and ARVI in adults
When planning to get vaccinated, you should remember that you can get immunity from the flu, with minimal risks of side effects, if you get a high-quality vaccine. It is very important to monitor the shelf life of the drug, its correct storage, transportation and compliance with standards.
Flu vaccination may be contraindicated:
- intolerance to those components that are part of the vaccine (chicken protein, preservatives);
- children's age up to six months;
- period up to 1 month after recovery from ARVI;
- signs of respiratory infection;
- presence of elevated body temperature;
- severe allergic reaction to a previous vaccine.
Contraindications
There are the following contraindications to vaccination with Influvac:
- acute infectious disease;
- stage of exacerbation of a chronic disease;
- hypersensitivity to the substances included in the vaccine;
- allergy to egg white, which is used in the production of the vaccine;
- first trimester of pregnancy;
- age up to 6 months;
- body temperature is above 37 degrees.
The effect of this vaccine on the fetus and pregnant women has not been sufficiently studied. The decision on the need to use this vaccine during lactation is made by the attending physician. For mild ARVI and acute intestinal disorders, the vaccine can be administered immediately after body temperature normalizes.
Flu vaccination methods
Immunization is the most effective method of preventing influenza. To protect yourself from respiratory diseases and complications caused by the penetration and spread of bacterial infections inside the body, you can resort to vaccination.
Today, vaccines may contain one of the following types of viral proteins:
- Weakened - viral cells will be alive, but will not be able to cause harm to the human body.
- Non-living, in other words inactivated viral fragments.
The last group contains three subspecies:
- whole cell: contain neutralized but not cleaved viral pathogens;
- split vaccines: here only fragments of the material that have undergone splitting are available;
- subunit: contain viral antigens, which makes it possible to more actively induce the production of protective antibodies by the human immune system.
The latest type of vaccine is considered the safest; it has a high degree of purification. In addition, it does not contain chicken protein, lipids, or those elements that most often cause allergies (especially in pediatric patients).
Subunit vaccines have a minimal likelihood of developing adverse reactions after vaccination; however, they form a stable immune response to influenza viruses.
These drugs are considered to be reference drugs on the pharmaceutical market. Moreover, the quality of vaccines does not change in any way depending on the country of origin: French and Russian are equally effective, only the domestic drug has a slightly lower cost.
We recommend reading: Flu Spanish
Side effects
After administration of the vaccine, the following side effects are possible:
- increased body temperature and general malaise, itching, rash, headache, redness and swelling at the site of vaccine administration - these symptoms disappear within 1-2 days;
- convulsions, vasculitis, neuralgia are manifestations that occur very rarely;
- anaphylactic shock – occurs extremely rarely.
Vaccination with Influvac may cause false positive results in serological tests when using the ELISA method, which are done to determine the presence of antibodies against hepatitis C and HIV-1, which is explained by the immune response to the vaccine.
Flu vaccine preparation for vaccination
The flu vaccine is necessary to familiarize the body as a whole and the immune system, in particular, with viral cells. Weakened virus particles cannot cause harm, causing the development of a full-fledged disease, but they enable the immune system to carry out a kind of “training” and remember a behavior pattern to combat such viral activity.
Part of vaccine production is to constantly monitor the strains of the virus that will spread in the current season and include them in immunization preparations.
To reduce the likelihood of possible negative manifestations, you should prepare for the procedure in advance:
- exclude the development of diseases of the respiratory organs, not only monitor the presence of any catarrhal symptoms, but also protect the body from hypothermia and communication with a large number of people, among whom there may be infected people;
- For about a week, regularly cleanse the nasal mucosa using irrigation with saline solutions (mandatory, after returning home);
- ensure that all exacerbations of chronic pathologies are in remission;
- Before vaccination, undergo an examination by a therapist, measure your body temperature, making sure that it is within normal physiological values.
After vaccination, there are several recommendations to keep in mind:
- the drug will be administered subcutaneously, after the procedure and for 1-2 days, you should not wet the injection site with water or scratch it to avoid infection;
- do not visit crowded places for two weeks to avoid infection with ARVI;
- refuse to visit the bathhouse or sauna for a while.