Can patients with epilepsy be vaccinated against COVID-19?
Sergei Georgievich Burd shares his expert opinion on whether patients with epilepsy can be vaccinated against COVID-19 .
According to information published by the International League Against Epilepsy (ILAE), there is currently no convincing evidence that epilepsy is associated with an increased risk of complications following COVID-19 vaccination. Moreover, it is noted that for people suffering from epilepsy, the risks of possible complications from the disease significantly exceed the risk of side effects after vaccination against COVID-19. The authors note that hyperthermia is possible after vaccination, which can lead to a decrease in the seizure threshold, so they recommend taking antipyretic drugs (for example, paracetamol) for 48 hours after vaccination. The authors ask patients to ensure that the medical personnel administering the vaccination are aware of the presence of epilepsy, as well as other medical information (allergic reactions, hyperthermia, current infectious diseases, taking medications that affect the immune system or anticoagulants, pregnancy, pregnancy planning and lactation).
In the UK, patients with epilepsy (“patients with chronic neurological conditions including epilepsy”) are included in a list of 9 priority groups for those who should receive the Covid-19 vaccine first.
The American Epilepsy Society (AES), citing the Epilepsy Foundation, also notes that there is currently no evidence of an increased risk of side effects or worsening of epilepsy in patients after vaccination against Covid-19. Like the experts of the International League Against Epilepsy, they note a possible increase in the threshold of convulsive readiness, as well as the risk of epileptic seizures associated with hyperthermia during vaccination. They also pay attention to the group of patients in whom epileptic seizures are associated with hyperthermia, recommending that this group of individuals discuss the benefits and risks of vaccination with an epileptologist.
Thus, epilepsy is not a contraindication to vaccination against Covid-19. Before vaccination, you must consult a physician for immediate access to vaccination.
Notes:
https://www.ilae.org/patient-care/covid-19-and-epilepsy/covid-19-vaccines-and-people-with-epilepsy/covid-19-vaccine
https://epilepsysociety.org.uk/latest/news/Covid-vaccine-reassurance
https://www.aesnet.org/about_aes/position_statements/covid-19/home
https://www.epilepsy.com/learn/covid-19-and-epilepsy/covid-19-vaccination
COVID-19 vaccination and people with epilepsy
COVID-19 vaccines are now being approved and made available to the public.
There is currently no evidence that epilepsy is associated with an increased risk of complications after COVID-19 vaccination, including the risk of seizures. For people with epilepsy, the risk of infection with COVID-19 and possible complications of the disease greatly outweighs the risk of side effects after receiving a COVID-19 vaccine. However, just like with vaccination against other diseases, fever may increase after vaccination against COVID-19. This may lower the seizure threshold in some patients. Taking antipyretics (such as paracetamol/acetaminophen) regularly for 48 hours after vaccination will help minimize the risk.
Before you get the COVID-19 vaccine, make sure the health care team administering the vaccine knows you have epilepsy and any other important medical information, such as:
- Allergy, especially allergic reactions to vaccine components;
- Previous allergic reactions to vaccines (such as the flu vaccine);
- Presence of fever or current infectious disease;
- Medicines you are currently taking, especially medications that affect the immune system (such as immunomodulators or immunosuppressants) and anticoagulants;
- Pregnancy, breastfeeding or planning pregnancy;
As with any vaccine, it is not recommended to get vaccinated against COVID -19 if you are allergic to any of the components of the vaccine. It is also not recommended to carry out the second stage of vaccination if you had an allergic reaction after the first.
If you have already been vaccinated against COVID -19, it is extremely important to continue to wear personal protective equipment and maintain social distancing.
Vaccination against COVID-19 currently reduces the risk of infection by up to 90%, depending on the vaccine, but vaccinated people can still transmit the disease.
Additional information can be found at the following links:
https://epilepsysociety.org.uk/vaccine
https://www.epilepsy.com/learn/covid-19-and-epilepsy/covid-19-vaccination
https://epilepsysociety.org.uk/news/Covid-vaccine-reassurance
https://livingwellwithepilepsy.com/2020/covid-19/covid-19-vaccine-and-epilepsy.html
rev. March 8, 2021
Who should not be vaccinated against COVID-19
(Material updated 06/20/2021)
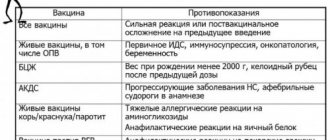
Contraindications to vaccination against COVID-19:
- hypersensitivity to the drug
- pregnancy and breastfeeding
- children under 18 years old
- ARVI symptoms less than 2 weeks ago
- other vaccinations given less than 30 days ago
- chronic diseases, epilepsy, stroke, autoimmune diseases, asthma, diabetes and others less than 2-4 weeks before remission or recovery (the decision is made by the attending physician)
- severe forms of allergic diseases
What is the difference between Russian COVID-19 vaccines
Who should not be vaccinated against coronavirus
Sputnik V. Contraindications
The instructions for use of the drug state that vaccination is contraindicated for people who are hypersensitive to any component of Sputnik V. This also applies to pregnant women and those who are breastfeeding or planning to conceive a child in the next three months - this, by the way, also applies to men. Children under 18 years of age are not yet vaccinated, since scientists do not have data on the safety and effectiveness of the drug for them.
People with ARVI symptoms are also not eligible for vaccination. They can only be given an injection after they have had an infection at least two weeks ago. Doctors will not vaccinate those who received any other vaccination later than 30 days ago, or who participated in post-registration studies of the vaccine.
The instructions for the drug also say that people with chronic liver and kidney diseases, severe endocrine system disorders, severe diseases of the hematopoietic system, epilepsy, as well as strokes and other diseases of the central nervous system, cardiovascular system, primary and secondary immunodeficiencies, autoimmune diseases, lung diseases, asthma. The same applies to patients with diabetes and metabolic syndrome, with allergic reactions and eczema.
It is noted that patients with acute infectious and non-infectious diseases and exacerbation of chronic diseases can be vaccinated only 2-4 weeks after recovery or remission.
There is an opportunity to carefully lift restrictions on COVID-19, Putin said
CoviVac. Contraindications
The instructions for use of the drug indicate the following contraindications for use. First of all, this is a serious post-vaccination reaction to any previous vaccination, accompanied by high fever (above 40°C), hyperemia, swelling or complications - collapse and shock-like state within 48 hours after vaccination, convulsions.
Also contraindicated are severe allergic reactions - anaphylactic shock, Quincke's edema, erythema multiforme, hypersensitivity.
Vaccination is also not recommended during pregnancy and breastfeeding. Children under 18 are also not vaccinated.
For chronic diseases of the liver, kidneys, endocrine system, cardiovascular system, bronchopulmonary system, gastrointestinal tract, immune system, vaccination can be prescribed with the permission of the attending physician.
EpiVacCorona. Contraindications
The instructions for use of the drug indicate that the vaccine is contraindicated for people who have hypersensitivity to the components of the drug (aluminum hydroxide, etc.).
Severe forms of allergic reactions, reactions and post-vaccination complications to a previous vaccine administration may also be a contraindication.
In case of acute infectious and chronic diseases, vaccinations are carried out no earlier than a month after recovery or remission.
Vaccination is also not recommended for immunodeficiency, malignant blood diseases and neoplasms, during pregnancy and breastfeeding, and for children under 18 years of age.
For chronic kidney diseases, liver diseases, endocrine system disorders, cardiovascular diseases, immunodeficiencies, autoimmune diseases, permission from the supervising physician will be required.
Can cancer patients get vaccinated?
On Monday, January 25, a number of media reported about new instructions from the Ministry of Health regarding recommendations for vaccination against coronavirus with restrictions for cancer patients. Meanwhile, this document was published on the department’s website on January 19. It describes possible adverse reactions from the Sputnik V drug and indicates what information about the patient needs to be collected before the injection.
Separately on the same day, director of the Gameley Center Alexander Gintsburg
clarified the warning for vaccinating Russians with malignant neoplasms. According to the scientist, cancer patients can also be vaccinated, but only after consultation with their doctor. A particularly dangerous time for cancer patients may be undergoing chemotherapy, which can reduce immune function.
The decision to vaccinate should be made by the attending physician for each individual cancer patient.
“Thus, the decision to vaccinate should be made by the attending physician for each specific cancer patient, which has nothing to do with the safety of the Sputnik V vaccine and does not indicate a ban on immunization of this group of patients,” Gunzburg clarified.
COVID-19 and pregnancy
On January 25, the Ministry of Health made publicly available recommendations for the treatment of pregnant women and newborns with COVID-19.
The document says that the coronavirus incidence among pregnant women is higher than in the population. Moreover, in general, such women are not prone to a more severe course of the disease, and are often asymptomatic. Pregnant women over 35 years of age with obesity, diabetes and high blood pressure suffer from infection worse.
The Ministry of Health stated that there is no evidence of vertical transmission of COVID-19 to a child
In addition, the Ministry of Health reported information related to the detection of RNA or antibodies to SARS-CoV-2 in umbilical cord blood, placental tissue, as well as the virus genome in the biological environments of newborns. However, there is currently no convincing evidence of vertical transmission of coronavirus from mother to fetus, the department emphasized.
Experts do not have reliable data on the isolation of this pathogen in breast milk, nor was it found in amniotic fluid or vaginal secretions. Cases of infection of a child from a mother were recorded only as a result of close contact of an infected woman or staff with the baby.
The Ministry of Health also clarified that only those newborns whose mothers are sick with coronavirus or are suspected of having an infection need to be tested for COVID-19. At the same time, the department does not recommend treating a sick child with existing drugs against the virus, since their safety and effectiveness for infants has not been proven.