In short:
There is information that the vaccine against pneumococcal infection can additionally protect against bacterial complications in those infected with coronavirus.
In detail:
The pneumococcal vaccine will not protect against coronavirus specifically. But! The vaccine will make it easier to get over the disease. With a moderate course of coronavirus infection against a background of weak immunity, pneumococcal pneumonia is often associated. Without help, the body is weakened and cannot cope on its own. It is then that the immunity developed after pneumococcal vaccination comes into effect.
| Germany faces shortage of pneumococcal vaccine due to COVID-19 harm reduction recommendations
Now let's figure out how and why it works
With the spread of coronavirus, the German Ministry of Health recommended that all people over 60 years of age be vaccinated against pneumococcal infection. This will not protect against coronavirus, but will help avoid complications from pneumonia, including deaths. 65-year-old Angela Merkel also decided to get vaccinated. It’s not just the elderly who are interested in the vaccine. This led to a shortage.
The Russian Ministry of Health does not yet provide such recommendations. Neither the older nor the younger generation. The World Health Organization (WHO) notes that vaccination against pneumococcal disease will not protect a person from coronavirus, but adds: “Vaccination against respiratory diseases is important to protect your health.”
Doctors say pneumococcal vaccination will not help prevent COVID-19
Vaccination against pneumococcus helps to avoid infection with COVID-19, says Kant IKBFU research professor Andrei Prodeus.
“This is an absolutely confirmed fact: those vaccinated against pneumococcus are less likely to become infected,” the specialist told the Izvestia newspaper. “Their immunity can resist any respiratory viruses, including SARS-CoV-2. In some regions of Russia, the pneumococcal vaccine is included in free regional programs.”
At the same time, immunologist Maria Polner and therapist Marina Kazakova are confident that the pneumococcal vaccine cannot affect COVID-19 infection. They told Gazeta.Ru about this. Honored Doctor of the Russian Federation, leading scientific editor of vrachu.ru Mikhail Kagan also agrees with them.
“Of course, antibodies to pneumococcus, the production of which are caused by such vaccines, have no effect on Covid,”
- the expert noted in a conversation with Gazeta.Ru. However, he said, the pneumococcal vaccine activates the innate immune system, which can more effectively fight off any germs.
“These activated mechanisms are the same for both pneumococcus and covid, and indeed for all microbes. The disadvantage is that while protection against pneumococcus with antibodies lasts for years, activation of innate immune factors lasts a short time - a maximum of 2-3 months,” Kagan explained.
He added that at the moment there is no direct evidence of the effectiveness of vaccinations against pneumococcal infection against COVID-19, despite a number of indirect factors that suggest this.
However, earlier, in August 2021, a scientific group from Italy, which included specialists from the geriatric departments of the Faculty of Medicine of the University of Padua and the Biomedical Campus in Rome, examined how vaccination against influenza and pneumococcus correlated with the results of PCR tests for COVID-19. The results of the scientific work were published in the scientific database PubMed.
The analysis found that adult and older respondents who received a pneumococcal vaccine in the past year were much less likely to test positive for coronavirus than their unvaccinated peers.
At the same time, vaccination against pneumococcal infection really helps to avoid complications from COVID-19, some Russian doctors are sure. As Maria Polner explained to Gazeta.Ru, not only with coronavirus, but also with any other acute respiratory viral infections, there is a high risk of complications in the form of bacterial respiratory tract infections, which are most often caused by pneumococcus.
“This is especially true for people with reduced immune defense,” the doctor emphasized. Prodeus, in turn, clarified that pneumococcus causes complications such as purulent otitis media, community-acquired pneumonia and sinusitis.
Vaccination against this bacterium can significantly improve the functioning of the barrier immunity of the mucous membranes, the doctor told Izvestia.
Pulmonologist Marina Kazakova said that there are two types of vaccines against pneumococcal infection. “There is a 13-valent vaccine, its use allows the formation of long-term immunity and memory cells. And there is a 23-valent polysaccharide vaccine that produces a pronounced immune response for about 5 years. Risk groups are recommended to administer both at intervals of 2 months to a year,” the expert explained to Gazeta.Ru.
According to infectious disease specialist Evgeniy Timakov, a 23-valent vaccine is suitable for adults, but children should get a 13-valent one. “The first acts on a larger number of subspecies of pneumococcus, but is less purified and can be more difficult to tolerate,” the doctor said in a conversation with Izvestia.
The chief epidemiologist of the Russian Ministry of Health, Nikolai Briko, meanwhile, told the publication that it is too early to say which vaccine is more effective in reducing mortality and the number of severe cases of coronavirus infection, since no one has yet conducted a comparative analysis of them within the framework of a pandemic. However, there are indeed recommendations that specifically state who is indicated for which drug.
“For children under two years of age, it is recommended to use the thirteen-valent vaccine. For people over 65 years of age, as well as patients with chronic diseases, we recommend starting with the same vaccine, and a year later being vaccinated with the twenty-three-valent one. The twenty-three-valent vaccine is recommended for healthy adults,” the specialist noted.
According to Nikolai Briko, the best result against coronavirus infection will be brought by combined vaccination – one that is effective against both pneumococcus and influenza. Such vaccinations reduce the number of deaths and the severity of the disease, due to the possible stimulation of innate immunity or any cross-immune reactions, the epidemiologist is convinced.
How exactly does the vaccine help?
Even if, as a result of COVID-19, the virus causes lung damage in a vaccinated person, it will most likely NOT be accompanied by a secondary pneumococcal infection. This means that there will be fewer complications, and the disease itself will pass more calmly. The pneumococcal vaccination works as a prevention of complications and can reduce their severity due to the presence of immunity.
Chairman of the Russian Respiratory Society, pulmonologist, academician of the Russian Academy of Sciences Alexander Chuchalin noted that the 13-valent pneumococcal vaccine “ can increase innate immunity”
:
“It has such properties, that is, here we are talking not only about the fight against pneumococcus, but immunity for coronavirus infection is a fundamental issue
».
The expert explained that “when a person suffers an infection such as coronavirus, a pronounced immunodeficiency state develops on the 14th–20th days from the onset of the disease”
.
What does it mean? The protective properties of the respiratory system become weak, and for pneumococcus, which inhabits the oropharynx, a favorable environment arises - it easily penetrates the lower respiratory tract. The pneumococcal vaccine is precisely the remedy that can protect in this situation.
A large population-based study showed that Prevenar 13
®
reduces the risk of hospitalization for community-acquired pneumonia in older people.
The risk of developing pneumococcal pneumonia or severe invasive forms of pneumococcal infection in adult patients is high. Currently, the resistance of the pathogen to antibiotics is progressively increasing, which causes certain difficulties in the treatment of pneumonia. Streptococcus pneumoniae, also known as pneumococcus, is the most common bacterial cause of CAP. Despite the population effect of childhood vaccination, vaccination of adults against pneumococcal infection remains relevant.
Pfizer Inc. presented data from a study on the effectiveness of routine vaccination with Prevenar 13® (pneumococcal thirteen-valent conjugate vaccine, PCV13) – reduction by 73%
risk of hospitalization for community-acquired pneumonia in adults aged 65 years and older. The results were published in the journal Clinical Infectious Diseases on May 22, 2021.
The study was conducted by Pfizer in collaboration with the University of Louisville School of Medicine, USA. The study design chosen, a population-based observational prospective case-control study with negative control, is considered a reliable type of observational study for assessing vaccine effectiveness in real-world practice.
It is important to note that the effectiveness of Prevenar 13® has been proven in routine vaccination settings, when people received the vaccine against pneumococcal infection according to the recommendations of their physicians.
“The new efficacy data further demonstrates that vaccinating adults aged 65 years and older against pneumococcal disease with PCV13 can help prevent hospitalization and save lives,” said Dr. Julio Ramirez, chief of the Division of Infectious Diseases at the University of Louisville School of Medicine and one of the authors of the study. “Specifically, based on the 73% vaccine efficacy observed in this study, we believe that vaccinating adults over 65 years of age with PCV13 will help prevent thousands of hospitalizations of community-acquired pneumonia.”
“These results complement the CAPITA clinical trial of immunization of adults against community-acquired pneumonia and confirm the effectiveness of PCV13 in a real-world immunization program involving high-risk individuals,” said Dr. Louis Jodard, President of the Department of Medical and Scientific Activities, Development and Clinical Development on vaccines Pfizer Inc. “These results, as well as the continued circulation of serotypes included in PCV13 among adults, support the importance of direct vaccination in this age group.”
About the Negative Control Case-Control Study
The study was conducted as a population-based observational prospective negative control case-control study among adults in Louisville, Kentucky, USA. The study recorded all cases of community-acquired pneumonia in adults living in this locality who sought hospitalization in the emergency department of one of the city's nine hospitals between April 1, 2015 and April 30, 2016. In this study, patients hospitalized with community-acquired pneumonia underwent both standard testing and a urine antigen test to identify pneumococcal serotype and compare it with vaccine-specific Prevenar 13® serotypes. Patients with pneumonia caused by a vaccine-specific pneumococcal serotype were defined as “cases,” and patients with PCV13 serotype-negative PFS were defined as “negative controls.”
Why population-based research data is important
Field studies, which analyze real-world data, can complement randomized clinical trials and provide additional information about how a drug works in routine medical practice.
Additional information about the study population
88% of study participants had at least one major risk factor for pneumococcal pneumonia, including chronic obstructive pulmonary disease (53%), coronary artery disease (35%), congestive heart failure (32%) and diabetes (32%) . 46% had immunodeficiency states, including those due to chronic kidney disease (23%) and cancer (19%). The average length of hospitalization for PFS was 6 days. 6.5% of patients with CAP died during the initial hospitalization, and another 12.7% died within the subsequent 30 days.
Pneumococcal infection in adults
Pneumonia (without or with bacteremia) is the most common form of pneumococcal infection in adults.
It should also be noted that in the Russian Federation, pneumococcus is the leading causative agent of bacterial meningitis among adults over 25 years of age: among etiologically deciphered cases, the incidence rate was 0.18-0.22 per 100 thousand population for pneumococcal meningitis, compared to 0.11-0 .03 for meningococcal meningitis and 0.01 for hemophilic meningitis. Among the age cohort of 25 years and older, the mortality rate for pneumococcal meningitis is 20-29%.
About Prevenar 13
.
Vaccine Prevenar 13®
(pneumococcal polysaccharide conjugate adsorbed vaccine, thirteen-valent). Registered in Kazakhstan in 2010, in the Russian Federation in 2011, in Belarus in 2012 for use in children. It became available for use in adults over 50 years of age in 2012, and in 2015 it was registered for use from 2 months. life and beyond without age limit
Indications for use
- prevention of pneumococcal infections, including invasive (including meningitis, bacteremia, sepsis, severe pneumonia) and non-invasive (community-acquired pneumonia and otitis media) forms of diseases caused by Streptococcus pneumoniae serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F from 2 months of life onwards without age restrictions:
— within the framework of the national calendar of preventive vaccinations;
- in individuals at increased risk of developing pneumococcal infection.
Vaccination is carried out within the framework of the national calendar of preventive vaccinations according to the approved dates, as well as for persons at risk for the development of pneumococcal infection: with immunodeficiency conditions, incl. HIV infection, cancer, receiving immunosuppressive therapy; with anatomical/functional asplenia; with a cochlear implant installed or planning to have this operation; patients with cerebrospinal fluid leakage; with chronic diseases of the lungs, cardiovascular system, liver, kidneys and diabetes; patients with bronchial asthma; premature babies; persons in organized groups (orphanages, boarding schools, army groups); convalescents of acute otitis media, meningitis, pneumonia; long-term and frequently ill children; patients infected with Mycobacterium tuberculosis; all persons over 50 years of age; Tobacco smokers.
Manufacturing company
1) Pfizer Ireland Pharmaceuticals, Ireland Grange Castle Business Park, Clondalkin, Dublin 22, Ireland
2) LLC "NPO Petrovax Pharm", Russian Federation 142143, Moscow region, Podolsk, p. Pokrov, st. Sosnovaya, 1
Limitations of use and effectiveness
Prevenar 13® does not protect against disease caused by S. pneumoniae serotypes not included in the vaccine. Prevenar 13® is approved for use in adults over 50 years of age in more than 100 countries. Prevenar 13® is approved in the EU and more than 40 other countries for use in adults 18 to 49 years of age.
Important Safety Information
Prevenar 13® should not be administered to patients with a known severe allergic reaction to any component of Prevenar 13® or to any vaccine containing diphtheria toxoid.
Children and adults who are immunocompromised (eg, due to HIV infection, leukemia) may have a reduced immune response.
In adults, the most common adverse reactions were pain, redness and swelling at the injection site, limited arm movement, fatigue, headache, muscle pain, joint pain, decreased appetite, vomiting, fever, chills and rash.
Premature infants may experience episodes of apnea after vaccination with any vaccine.
The most commonly reported serious adverse reactions in infants and toddlers were bronchiolitis (lung infection) (0.9%), gastroenteritis (inflammation of the stomach and small intestine) (0.9%), and pneumonia (0.9%).
In children 6 weeks to 17 years of age, the most common adverse reactions were weakness, redness or swelling at the injection site, irritability, decreased appetite, decreased or increased sleep duration, and fever.
Pfizer: Building a Healthier World Together
Through innovation and global resources, Pfizer works to improve the health and well-being of people at every stage of life. We strive to set high standards for the quality and safety of our drug research, development and production. The company's product portfolio includes pharmaceuticals, including vaccines, as well as world-famous vitamins and other health-promoting products.
Every day, Pfizer employees work in developed and developing countries to improve the prevention and treatment of some of today's most serious diseases. Living up to its commitment as the world's leading biopharmaceutical company, Pfizer partners with healthcare professionals, governments and communities to ensure and expand access to reliable, quality health care around the world.
For more than 160 years, Pfizer has been committed to improving the lives of those who count on us.
What is pneumococcus?
Pneumococci are microbes that inhabit our upper respiratory tract. They can lead to a variety of diseases when the body’s immunity is weakened. With normal immunity and the absence of provoking factors, such as hypothermia or contact with sick people, these microbes do not spread from their habitats.
About numbers
In 2005, the World Health Organization made calculations, and the following picture emerged: 1.6 million people die every year
from pneumococcal infection, of which
800,000 are children under 2 years old and 200,000 are children from 2 to 5 years old.
By the end of 2013, the pneumococcal vaccine had been introduced in 103 countries.
Immunization coverage
reached 25% of the population
. By order of the Ministry of Health of the Russian Federation No. 125 dated March 21, 2014, vaccination against pneumococcal infection was introduced into the National Calendar of Preventive Vaccinations.
World experience has shown that mass vaccination
reduces the incidence of pneumococcal meningitis
by more than 80% and the incidence of all pneumonia and otitis by more than a third.
Pneumococcal infections
Symptoms:
- The infection is caused by pneumococcal bacteria, of which several dozen subtypes are known. The infection can manifest itself as relatively mild - acute respiratory infections, otitis media, or the development of pneumonia, purulent pneumococcal meningitis, and sepsis.
- Pneumococcal pneumonia usually occupies a leading place among bacterial pneumonias. Severe pneumococcal pneumonia is often associated with the development of sepsis (bacteria entering the blood);
- the mortality rate may increase in patients with age, as well as in the presence of concomitant chronic diseases and metabolic disorders;
- the symptoms of pneumococcal meningitis do not differ from other bacterial meningitis (meningococcal and CHIB). Mortality from pneumococcal meningitis is high, especially in young children and the elderly;
Treatment is carried out with the use of antibiotics, but may not always be successful. Many subtypes of pneumococcus are resistant to a wide range of antibiotics, making treatment very difficult.
Epidemiology and vaccination
- Pneumococci are transmitted from person to person, for example, by coughing. The source of infection can even be a carrier of pneumococci in the nasopharynx, who does not have any clinical manifestations.
- Pneumococcal infections can affect all age groups, but the most severe course is observed in children of the first years of life, the elderly and people with various chronic diseases.
- Pneumococcal infections are widespread in countries where mass vaccination against pneumococcal infection of children and adults at risk is not carried out. About 100 subtypes of pneumococcus have been identified in the world, but about 30 of them are the most common and antibiotic-resistant.
- Vaccination against pneumococcal disease is generally given to reduce the incidence of respiratory tract infections and other severe pneumococcal diseases in children and adults.
- There are two types of pneumococcal vaccines – polysaccharide and conjugate. These vaccines differ in the number of pneumococcal serotypes included and in the possibility of their use in children in the first two years of life. These vaccines can be used individually or sequentially to expand serotype coverage. For children from two years of age from high-risk groups, people with severe immune disorders, as well as people over 65 years of age, sequential use of these two types of vaccines is recommended.
Adverse reactions
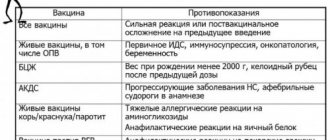
Pneumococcal vaccines are inactivated (killed) and are well tolerated. Expected reactions, according to the instructions for use, are redness, induration/swelling, pain at the injection site, and sometimes increased body temperature.
Contraindications
A permanent contraindication is a very strong reaction to the previous dose of this vaccine and established hypersensitivity to its components. Temporary contraindications are standard for inactivated vaccines.
Show sources
Sources
What vaccines are used?
There are polysaccharide and conjugate vaccines. Polysaccharide ones contain a larger number of strains (varieties) of pneumococci, while conjugated ones are superior in terms of age-specific breadth of application and duration of protection.
In our center, we more often use conjugate drugs - those that are used from 2 months of age and provide long-term immunity, often lifelong.
Pneumococcal vaccines: Prevenar-13 or Synflorix.
They protect against invasive forms of the disease - peritonitis, endocarditis, meningitis, pneumonia, sepsis and form long-term immunological memory. In other words, the body knows, remembers and is ready for defense.
Who is Menactra for?
Menactra is designed to vaccinate children from 9 months of age and adults up to 55 years of age. Children aged 9 months to 2 years are vaccinated twice with a break of at least 3 months. Children over 2 years old - once.
Menactra forms long-term immunity to meningococcal infection. The official website of the drug states that revaccination is possible no less than 4 years after the initial vaccination, at the age of over 15 years and with a continuing risk of getting sick. But since the birthplace of the vaccine - the United States - is a country of mass immunization of the population against this disease, revaccination is not done there.