Do I need to get a flu shot when planning a pregnancy? — MEDSI
Table of contents
- Flu vaccine and pregnancy planning
- Immune system during pregnancy
- Danger of the virus for pregnant women
- What vaccinations should I get when planning?
- Vaccine options
- When it is contraindicated
- Is it possible to get vaccinated during pregnancy?
- Adverse reactions
- Advantages of carrying out the procedure at MEDSI
Pregnancy planning should begin at least six months before conception. And getting a flu shot is included in the list of necessary actions.
Flu vaccine and pregnancy planning
A flu shot should be given before conception for several reasons:
- So that the woman’s body has time to develop antibodies to the disease
- To make it easier for the expectant mother to endure vaccination while her immunity is not yet weakened
- To avoid harm to the developing baby
Immune system during pregnancy
During pregnancy, a woman's immune system weakens to allow the fetus to develop. Otherwise, it will be rejected by the body as a foreign body. But due to lower immunity, the expectant mother is more at risk of contracting diseases than others.
Danger of the virus for pregnant women
A pregnant woman can easily become infected with the flu. It is most dangerous due to the likelihood of complications such as:
- Exacerbation of chronic diseases
- Miscarriage
- Development of heart defects or other organ defects in the fetus
- Premature birth
During this period, the introduction of medications into the body of the expectant mother is limited, since not all of them are safe for the developing baby.
What vaccinations should I get when planning?
Since it is not always possible to reliably find out whether the expectant mother has previously been vaccinated against certain diseases, when planning to bear a child, it is necessary to receive vaccinations from the following list:
- In seven months:
- Hepatitis B – done three times in stages
- Rubella
- Parotitis
- Measles
- Chicken pox
- Flu
- Tetanus
- Diphtheria
- Polio
If a woman becomes infected with a similar disease while carrying a fetus, there is a risk that it will develop developmental disorders and pathologies. There is also the possibility of miscarriage.
Vaccine options
A flu shot is required when planning a pregnancy. There are several drug options. Some of them contain elements of the destroyed pathogen, while others contain a living but weakened virus.
It is recommended to use the newest vaccine options. They appear in the fall of every year.
When it is contraindicated
Vaccination against this disease cannot be used in the following cases:
- First trimester of pregnancy
- The person is allergic to antibiotics and chicken eggs (in this case, there are types of drugs that can be used)
- The patient is already infected
Is it possible to get vaccinated during pregnancy?
The vaccine can be used in the second and third trimester of pregnancy. It is not prescribed in the first twelve weeks.
Adverse reactions
- Severe headache
- Discomfort and redness of the skin at the injection site
- Nausea
Advantages of carrying out the procedure at MEDSI
- Only modern high-quality vaccines are used - the safest and most effective
- A specialist can visit your home
- Make an appointment by phone 8 (495) 7-800-500
Why do pregnant women need a flu vaccination?
25.11.2015
Vaccination of pregnant women against influenza has been routinely performed in some countries of Europe and America for more than 20 years, and its immunological effectiveness reaches 70-85%. Since 2014, in Russia, flu vaccination of pregnant women has been included in the National Vaccination Calendar. Why is it so important to vaccinate expectant mothers against the flu, what is the danger of infection with influenza and acute respiratory infections during pregnancy, does vaccination have a negative effect on the course of pregnancy, how does it affect the development of the fetus? These and other questions were answered during an information seminar for parents by Mikhail Kostinov, head of the laboratory of vaccine prevention and immunotherapy of allergic diseases of the Federal State Budgetary Institution Research Institute of Vaccines and Serums named after. I.I. Mechnikov”, professor, doctor of medical sciences, expert of the portal “Vaccination Specialists”.
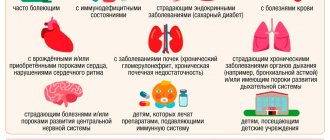
According to WHO recommendations, priority groups for influenza vaccination are pregnant women and children aged 6 months and older. Why is such attention paid to these particular categories of the population, and what is the rationale for vaccinating pregnant women against influenza? The fact is that maternal infection with the influenza virus often causes complicated pregnancy, intrauterine (antenatal) fetal death, the development of congenital pathologies in the child, damage to the central nervous system, as well as disorders of neuropsychic and physical development in children in the first years of life.
There are many studies and literature about the danger of this infection for expectant mothers. Mikhail Kostinov relies on a large-scale study by Russian scientists from the Chita Medical Academy, which they conducted during the influenza pandemic in 2009-2010. During the study, scientists observed a group of pregnant women with acute respiratory infections (ARI) and influenza, as well as a group of pregnant women who did not have these diseases. The study found that pregnant women who have had the flu often develop complications during pregnancy. Thus, when influenza and ARI were combined in the first trimester of pregnancy, placental insufficiency was observed in 47% of cases, in the second trimester - in 78% of cases, and in the third trimester this figure was 92%. These rates were significantly higher than in women who did not have the flu during pregnancy. For comparison: in women without ARI in the third trimester, placental insufficiency was observed in 44% of cases versus 92% of those who became ill. The danger lies in the fact that with placental insufficiency, while still in the womb, the child does not receive sufficient nutrition (including for the development of the nervous system), and metabolic processes are disrupted. Fetal hypoxia during ARI and influenza during pregnancy, depending on which trimester the infection occurred (first, second and third), develops in 70%, 67%, 80% of cases, respectively. When fetal hypoxia occurs, oxygen access becomes difficult, which subsequently affects the child’s neuropsychic development. Preeclampsia is also fraught with complications for both the mother and the fetus. When suffering from influenza, they actively develop in the first and second trimester in 25% and 30% of cases (at the time of fetal formation) and decrease to 15% in the third trimester.
Influenza and acute respiratory infections also complicate the course of labor. According to the study, in women who have had ARI and influenza in the first trimester of pregnancy, premature birth is observed in 12% of cases (only 4% in those who were not sick). Untimely rupture of amniotic fluid is typical for women who suffered ARI and influenza in the third trimester - 24%. A defect in the placenta and tight attachment of the placenta was noted in 6% of cases when the disease was experienced in the third trimester, which is significantly higher than in women who did not suffer from these diseases.
Doctors are also concerned about the development of children in the early neonatal period. If a child is born from a mother who suffered from ARI and influenza in the first trimester of pregnancy, then in 86% of cases a disorder of adaptation is observed (in 46% of children born to mothers who did not suffer from ARI and influenza); in the second trimester, a disorder of adaptation is recorded in 73% of cases, in the third – in 76%. One of the dangerous consequences of influenza and acute respiratory infections during pregnancy that affects the child is cerebral ischemia, which can cause disturbances in the neuropsychic development of children. When expectant mothers suffered ARI and influenza, cerebral ischemia was recorded in 76% of cases in the first trimester, and in 62% in the second trimester. These figures are more than twice as high as those for children born to mothers who did not have ARI or influenza during pregnancy.
According to the study, influenza and acute respiratory infections during pregnancy can also cause congenital abnormalities of the fetus. During a study conducted by Chita scientists (307 children were examined), among such anomalies, in particular, a ventricular septal defect was noted: when mothers fell ill with influenza and ARI in the first trimester - 4.8%, in the second - 1.4%, in the third – 2.4%. One case of bilateral clubfoot in a child was identified, while the mother suffered in the first trimester not from the flu, but from an ARI of the first severity. Also noted were one case of syndactyly, a congenital abnormality of the limbs characterized by the fusion (full or partial) of two or more fingers or toes, and two cases of polydactyly, an abnormality characterized by a greater than normal number of fingers or toes. Two children had thymus enlargement syndrome and a number of other anomalies.
The studies carried out allowed us to draw conclusions about the frequency of congenital anomalies per 1 thousand births. According to the study, in Russia during the influenza pandemic in 2009-2010, 58-59 congenital anomalies were recorded per 1 thousand births. Let us note that on average in Russia, according to the national guidelines for obstetrics and gynecology for 2007, influenza and ARI in pregnant women occur 5-6 congenital anomalies per 1 thousand. According to WHO, in the world - 4-6 cases per 1 thousand born. Thus, during influenza epidemics and pandemics, the risk of developing congenital anomalies increases tenfold.
Many expectant mothers believe that the flu can be cured with conventional antiviral drugs. But it is worth noting that the role of antiviral therapy against influenza in pregnant women is still unknown. There is no evidence that the drugs recommended for pregnant women (in particular, Tamiflu and Relenza) are completely safe. The fact is that the safety of drugs recommended for pregnant women must be confirmed by a large-scale study on volunteers, which is difficult to do under current conditions, since such studies have not been conducted. Therefore, the strategy of preventing influenza in pregnant women using vaccines with a proven safety record is preferable to using antiviral drugs with unknown effects.
Today, various types of vaccines are used to vaccinate pregnant women against influenza:
– split vaccines, which have been used all over the world for vaccination of pregnant women for decades;
– subunit vaccines – vaccines of the latest generation, in which maximum purification of antigens from toxic impurities is achieved;
– adjuvanted vaccines, which are used to quickly create full immunity against influenza (with a pandemic strain of the virus, two doses of conventional vaccines or one adjuvanted vaccine are required to create full immunity).
Also in Russia, an immunoadjuvant vaccine is used, which includes an immunomodulator that has an adjuvant effect.
As always, the safety of vaccines raises many questions; many expectant mothers fear that vaccinations will negatively affect the course of pregnancy and affect the child. Vaccine safety requirements in Russia are high and confirmed by many studies. As part of this seminar, research data on the safety of the widely used influenza vaccines Grippol Plus and Agrippal were presented. According to international standards, several groups of women took part in the study: pregnant women vaccinated with Grippol Plus or Agrippal (contain three strains of influenza virus - A (H1N1), A (H3N2) and type B virus); pregnant women vaccinated with the monoinfluenza vaccine Mono Grippol Plus (one strain A (H1N1), and the “placebo” group - women who received saline solution. Vaccination, according to international recommendations, was carried out in the second and third trimester of pregnancy. During the study, observations were also carried out for children born to these women.
The first indicator when assessing the safety of vaccination in pregnant women is the development of local reactions (swelling at the injection site, redness, pain at the injection site, etc.). Local reactions to the drug Grippol Plus were recorded in 13.5% of cases, compared with the placebo group - 3.3%. Such reactions occurred 5-6 hours after the injection and completely disappeared after 2-2.5 days, without the use of additional medications. In the dynamics of the vaccination process (7-30 days after injection), general somatic changes were generally observed comparable to the “placebo” group.
The next parameter for assessing vaccine safety is assessing the dynamics of changes in basic biochemical parameters. In this case, the indicators in pregnant women vaccinated with Grippol Plus and in the placebo group were also comparable and did not go beyond the normal range.
The indicator of perinatal screening of fetal development is very important. As the study found, vaccination had no effect on the intrauterine development of the fetus.
The next parameter for assessing the safety of vaccines in the study was ultrasound monitoring of the fetus during intrauterine development. In this study, at 22-23 weeks of pregnancy, the circumference of the head, abdomen, hip development, and so on is determined. Among those vaccinated with vaccines and the placebo group, no significant differences were found in all parameters. The same study was conducted at 35 weeks of pregnancy and also showed no effect of vaccination on intrauterine development. The data presented indicate that vaccination of pregnant women against influenza does not affect the course of pregnancy.
Next, a group of scientists monitored the outcome of pregnancy and childbirth in all studied groups of women (vaccinated with Mono Grippol Plus, Grippol Plus, Agrippal, and the placebo group). The rate of “term birth” was almost the same in all groups – 90%, 96%, 89% and 85%, respectively. Similar data were also obtained for the “miscarriage” indicator in the groups - this parameter did not exceed 2%. Preterm birth was also similar in all groups. Children were born without pathologies in the first group (Mono Grippol Plus) - in 88.4% of cases, in the second group (Grippol Plus) - in 92%, in the third group (Agrippal) - in 87.5%, in the placebo group (saline solution) – in 85.4%. The same indicators of perinatal CNS damage were recorded in all groups. The syndrome of intrauterine infection was also similar in all groups and ranged from 4.6% to 4.9%.
Scientists also observed the postnatal development of children. During the newborn period, Apgar scores of 8-9 were recorded virtually proportionately in all groups: 92.1%, 87.5%, 80.9% and 94.3%. The neuropsychic development of children in the first 6 months was assessed together with neurologists. 81.6% developed without disturbances when their mothers were vaccinated with Mono Grippol Plus, 83.3% of children from mothers vaccinated with Grippol Plus, Agrippal - 78.6% and saline - 77.1%.
Very often, when explaining refusal to vaccinate during pregnancy, women refer to the fact that breastfeeding will be disrupted in this case. These studies refute this. The breastfeeding rate at birth was 100% (group vaccinated with Mono Grippol plus), 85.4% (Grippol plus), 92.9% (Agrippal), 100% (in the placebo group).
As for the harmonious physical development of children, children in all groups had average harmonic development and above average harmonic development. Thus, based on the study data, we can draw an unambiguous conclusion: vaccination of pregnant women did not have any negative impact on the development of children.
When conducting vaccine trials, it is important to know how the immunity of pregnant women is formed during vaccination. It is believed that the protective level of antibodies is formed at a rate equal to or more than 1:40. And the vaccine is considered immunogenic if at least one of the parameters for assessing the immunogenicity of vaccines (seroprotection, seroconversion and seroconversion factor) corresponds to this indicator. These studies showed that after a month, 75% of those vaccinated, for example, with Grippol Plus had a sufficient level of antibodies, for example, to the H1N1 influenza strain. After 3-4 months after vaccination, the percentage of protected pregnant women exceeds 67%.
How long can post-vaccination immunity to the influenza virus last in newborns? In almost 70% of cases, newborn children have transplacental antibodies, that is, transmitted to them from their mothers. And if, for example, the mother has a seropositivity level of antibodies of 1:20, then the baby will have the same level. After three months, the conditional level of protection against influenza remains in 50% of children; after six months, only a quarter of newborns have protective antibodies. Note that, according to international recommendations, a level of protection with an antibody titer level of 1:40 is considered effective. This level of protection after six months is not recorded in children who were born to mothers vaccinated against influenza.
That is why the National Vaccination Calendar recommends vaccinating children against influenza starting at 6 months, since by this time maternal antibodies transferred to the child are lost, and vaccination makes it possible to protect him from influenza.
Many parents have asked whether it is possible to get a flu vaccine immediately after birth. Vaccines are safe, but at the moment there is no vaccine in the world that could provide immunity against influenza in newborns.
As the data from the studies reviewed during the seminar and many others conducted in various countries show, vaccination of pregnant women against influenza is absolutely safe if the doctor adheres to the recommendations that exist for each vaccine, takes into account contraindications, etc. The vaccines themselves do not affect the metabolic exchanges that occur in the body of pregnant women and do not affect the homeostasis of pregnant women. All vaccines are immunogenic and provide full immunity, which protects a woman from influenza virus strains during pregnancy and for another six months after the birth of a child. As for the effect of vaccination on the development of a child, it has been proven that during intrauterine development and after the birth of a child, vaccination of pregnant women has no effect negative impact on children.