Post-vaccination complications
Excessive toxic reactions are regarded as post-vaccination complications if they develop in the first three days after vaccination and are characterized by a pronounced disturbance in the child’s condition (temperature rise above 39.5°C, chills, lethargy, sleep disturbance, anorexia, possibly vomiting, nosebleeds and etc.) and are stored for 1-3 days. Typically, such post-vaccination complications develop after the administration of DTP, Tetracoc, live measles vaccine, influenza split vaccines, etc. In some cases, hyperthermia may be accompanied by short-term febrile convulsions and hallucinatory syndrome.
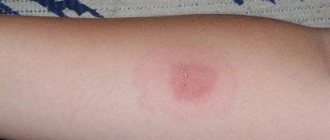
Post-vaccination complications that occur in the form of allergic reactions are divided into local and general. The criteria for a local post-vaccination complication are hyperemia and swelling of tissues that extend beyond the area of the nearest joint or to an area of more than 1/2 of the anatomical zone at the site of vaccine administration, as well as hyperemia, swelling and pain that persist for more than 3 days, regardless of size. Most often, local allergic reactions develop after the administration of vaccines containing aluminum hydroxide sorbent (DTP, Tetrakok, anatoksins).
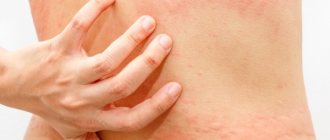
Among post-vaccination complications, there are also common allergic reactions: anaphylactic shock, urticaria, Quincke's edema, Lyell's syndrome, Stevens-Johnson syndrome, multimorphic exudative erythema, manifestation and exacerbation of bronchial asthma and atopic dermatitis in children. Immunization can cause the initiation of immune complex post-vaccination complications - serum sickness, hemorrhagic vasculitis, periarteritis nodosa, glomerulonephritis, thrombocytopenic purpura, etc.
Post-vaccination complications with an autoimmune mechanism of development include lesions of the central and peripheral nervous system (post-vaccination encephalitis, encephalomyelitis, polyneuritis, Guillain-Barré syndrome), myocarditis, juvenile rheumatoid arthritis, autoimmune hemolytic anemia, systemic lupus erythematosus, dermatomyositis, scleroderma, etc.
A peculiar post-vaccination complication in children in the first six months of life is a shrill cry, which is persistent (from 3 to 5 hours) and monotonous. Typically, a high-pitched scream develops after administration of the pertussis vaccine and is caused by an associated change in microcirculation in the brain and an acute attack of intracranial hypertension.
The most severe post-vaccination complications in terms of their course and consequences are the so-called vaccine-associated diseases - paralytic poliomyelitis, meningitis, encephalitis, the clinical symptoms of which do not differ from those of diseases with a different mechanism of occurrence. Vaccine-associated encephalitis can develop after vaccination against measles, rubella, and DTP. The likelihood of developing vaccine-associated meningitis after receiving the mumps vaccine has been proven.
Post-vaccination complications after administration of the BCG vaccine include local lesions, persistent and disseminated BCG infection. Among the local complications, the most common are axillary and cervical lymphadenitis, superficial or deep ulcers, cold abscesses, and keloid scars. Among the disseminated forms of BCG infection, osteitis (ostitis, osteomyelitis), phlyctenular conjunctivitis, iridocyclitis, and keratitis have been described. Severe generalized post-vaccination complications usually occur in children with immunodeficiency and are often fatal.
Post-vaccination reactions and complications – what do you need to know?
30.11.2015
What is considered a post-vaccination complication, why most reactions to vaccinations are not post-vaccination complications, what should doctors do if post-vaccination complications are detected. Official regulations set out fundamental provisions on these issues.
Post-vaccination complications. Registration, accounting and notification
In accordance with the Federal Law of the Russian Federation “On Immunoprophylaxis of Infectious Diseases”, post-vaccination complications (PVC) include severe and (or) persistent health problems due to preventive vaccinations, namely:
- anaphylactic shock and other immediate allergic reactions; serum sickness syndrome;
- encephalitis, encephalomyelitis, myelitis, mono(poly)neuritis, polyradiculoneuritis, encephalopathy, serous meningitis, afebrile convulsions, absent before vaccination and recurring within 12 months after vaccination;
- acute myocarditis, acute nephritis, thrombocytopenic purpura, agranulocytosis, hypoplastic anemia, systemic connective tissue diseases, chronic arthritis;
- various forms of generalized BCG infection.
Information about post-vaccination complications is subject to state statistical recording. When establishing a diagnosis of PVO, suspicion of PVO, as well as an unusual vaccine reaction during active observation during the vaccination period or when seeking medical help, the doctor (paramedic) is obliged to:
- provide the patient with medical care, and, if necessary, ensure timely hospitalization in a hospital where specialized medical care can be provided;
- register this case in a special accounting form or in the infectious diseases register on specially designated sheets of the register. Necessary clarifications and additions are subsequently made to the journal.
All data about the patient is recorded in detail in the appropriate medical documentation. Namely: the history of the development of a newborn, the history of the development of a child, the child’s medical record, the medical record of an outpatient, the medical record of an inpatient, as well as the emergency medical call card, the card of the person who applied for rabies treatment and the certificate of preventive vaccinations.
About uncomplicated isolated cases of strong local reactions (including swelling, hyperemia > 8 cm in diameter) and strong general reactions (including temperature > 40 C, febrile convulsions) to vaccination, as well as mild manifestations of skin and respiratory allergies, higher health authorities are not informed. These reactions are recorded in the child's developmental history, the child's or outpatient's medical record, the immunization certificate, and the clinic's immunization log.
When a diagnosis of PVO is made or suspected, the doctor (paramedic) is obliged to immediately inform the chief physician of the healthcare facility. The latter, within 6 hours after establishing a preliminary or final diagnosis, sends information to the city (district) center of state sanitary and epidemiological supervision. The head of the healthcare facility is responsible for the completeness, accuracy and timeliness of recording diseases suspected of air defense, as well as for prompt reporting of them.
The territorial center of state sanitary and epidemiological supervision, which has received an emergency notification about the development of air defense (or suspicion of air defense), after registering the information received, transfers it to the center of state sanitary and epidemiological supervision in the constituent entity of the Russian Federation on the day the information is received. The center of state sanitary and epidemiological supervision is also provided with information about series in which the frequency of development of strong local and/or general reactions exceeds the limits established by the instructions for use of the drugs.
Investigation of post-vaccination complications
Each case of complication (suspected complication), requiring hospitalization, or resulting in death, must be investigated by a commission of specialists (pediatrician, therapist, immunologist, epidemiologist, etc.) appointed by the chief physician of the regional state sanitary and epidemiological supervision in a constituent entity of the Russian Federation. When investigating complications after BCG vaccination, a TB doctor must be included in the commission.
When conducting an investigation, it should be borne in mind that there are no pathognomonic symptoms that would allow each specific case to be unambiguously considered a post-vaccination complication or an unusual reaction. And clinical symptoms such as fever, intoxication, neurological symptoms, various types of allergic reactions, incl. immediate type, may be caused not by vaccination, but by a disease that coincided in time with the vaccination. Therefore, each case of the disease that developed in the post-vaccination period, and is interpreted as a post-vaccination complication, requires careful differential diagnosis with both infectious (ARVI, pneumonia, meningococcal and intestinal infections, urinary tract infections, etc.) and non-infectious diseases (spasmophilia, appendicitis, intussusception, ileus, brain tumor, subdural hematoma, etc.) using instrumental (radiography, EchoEG, EEG) and laboratory (blood biochemistry with determination of electrolytes, including calcium, cerebrospinal fluid cytology, etc.) research methods, based on the clinical symptoms of the disease.
The results of a long-term analysis of deaths that developed in the post-vaccination period, carried out by the State Medical Inspectorate named after. L.A. Tarasevich, indicate that the vast majority of them were caused by intercurrent diseases (a disease detected against the background of an existing underlying disease and not being a complication of it). However, doctors, taking into account the temporary connection with the vaccine, diagnosed a “post-vaccination complication”, and therefore no etiotropic therapy was prescribed, which in some cases led to a tragic outcome.
Information indicating the possibility of a connection between post-vaccination complications and the quality of the administered vaccine:
- the development of complications is recorded in persons vaccinated by different medical workers after the administration of a vaccine of the same series or a vaccine from the same manufacturer,
- a violation of the temperature regime for storing and/or transporting the vaccine was detected.
Information indicating technical errors:
- PVOs develop only in patients vaccinated by a single healthcare worker;
Technical errors are caused by violation of the rules for storage, preparation and administration of medical immunobiological preparations, in particular: incorrect choice of location and violation of the technique for administering the vaccine; violation of the rules for preparing the drug before its administration: using other drugs instead of a solvent; diluting the vaccine with the wrong volume of diluent; contamination of the vaccine or diluent; improper storage of the vaccine - long-term storage of the drug in diluted form, freezing of adsorbed vaccines; violation of the recommended dose and schedule of immunization; using unsterile syringes and needles.
If a technical error is suspected, it is necessary to check the quality of work of the medical worker performing vaccination, provide him with additional training, and also evaluate the sufficiency and results of the metrological examination of the material and technical base: refrigerators may need to be replaced, disposable syringes are insufficient, etc.
Information indicating the patient's health characteristics:
- the appearance of stereotypical clinical manifestations after the administration of different series of the vaccine in patients vaccinated by different medical workers with a general history and clinical signs of the disease:
- the presence of hypersensitivity to the components of the vaccine in the form of allergic reactions in the anamnesis;
- immunodeficiency state (in case of vaccine-associated diseases after administration of live vaccines);
- history of decompensated and progressive lesions of the central nervous system, convulsive syndrome (in case of development of neurological reactions to DPT)
- the presence of chronic diseases that may worsen in the post-vaccination period.
Information indicating that the disease is not related to vaccination:
- identifying the same symptoms of the disease in vaccinated and unvaccinated people;
- unfavorable epidemic situation in the environment of the vaccinated person - close contact with infectious patients before or after vaccination can lead to the development of an acute disease, which coincides in time with the post-vaccination process, but is not associated with it.
Below are some clinical criteria that can be used in the differential diagnosis of post-vaccination complications:
- general reactions with fever, febrile convulsions to the administration of DPT and ADS-M appear no later than 48 hours after vaccination;
- reactions to live vaccines (except for immediate allergic reactions in the first few hours after vaccination) cannot appear earlier than the 4th day and more than 12 - 14 days after the administration of measles and 30 days after the administration of OPV and mumps vaccines;
- meningeal phenomena are not typical for complications after administration of DPT vaccine, toxoids and live vaccines (with the exception of mumps vaccine);
- encephalopathy is not typical for reactions to the administration of mumps and polio vaccines and toxoids; it extremely rarely occurs after DTP vaccination; the possibility of developing post-vaccination encephalitis after vaccination with DTP vaccine is currently disputed;
- the diagnosis of post-vaccination encephalitis requires, first of all, the exclusion of other diseases that may occur with cerebral symptoms;
- Facial neuritis (Bell's palsy) is not a complication of OPV and other vaccines;
- immediate allergic reactions develop no later than 24 hours after any type of immunization, and anaphylactic shock - no later than 4 hours;
- intestinal, renal symptoms, heart and respiratory failure are not typical for complications of vaccination and are signs of concomitant diseases;
- catarrhal syndrome may be a specific reaction to measles vaccination if it occurs no earlier than 5 days and no later than 14 days after vaccination; it is not typical for other vaccines;
- arthralgia and arthritis are characteristic only of rubella vaccination;
- Vaccine-associated poliomyelitis develops within 4 to 30 days after immunization in vaccinated people and up to 60 days in contact people. 80% of all cases of the disease are associated with the first vaccination, while the risk of developing the disease in immunodeficient individuals is 3-6 thousand times higher than that in healthy people. VAP is necessarily accompanied by residual effects (flaccid peripheral paresis and/or paralysis and muscle atrophy);
- lymphadenitis caused by the BCG vaccine strain usually develops on the side of the vaccine. The process usually involves the axillary, and much less frequently, the sub- and supraclavicular lymph nodes. A distinctive sign of a complication is the absence of pain in the lymph node upon palpation; the color of the skin over the lymph node is usually not changed;
- criteria to suggest the BCG etiology of osteitis are the age of the child from 6 months to 1 year, the primary localization of the lesion at the border of the epiphysis and diaphysis, a local increase in skin temperature without hyperemia - “white tumor”, the presence of swelling of the nearest joint, muscle rigidity and atrophy limbs (with appropriate localization of the lesion).
When conducting an investigation, information received from the patient or his parents is of significant assistance in making a diagnosis. These include data from the updated medical history of the patient, his state of health before vaccination, the time of appearance and nature of the first symptoms of the disease, the dynamics of the disease, pre-medical treatment, the presence and nature of reactions to previous vaccinations, etc.
When investigating any case of a post-vaccination complication (suspected complication), the place of distribution of the advertised series should be asked about possible unusual reactions after its use and the number of doses vaccinated (or used). In addition, it is necessary to actively analyze the appeal for medical care of 80 - 100 people vaccinated with this series (with inactivated vaccines - within the first three days, with live viral vaccines administered parenterally - within 5 - 21 days).
With the development of neurological diseases (encephalitis, myelitis, polyradiculoneuritis, meningitis, etc.), in order to exclude intercurrent diseases, it is necessary to conduct serological studies of paired sera. The first serum should be taken as early as possible from the onset of the disease, and the second - after 14 - 21 days.
In sera, antibody titers to influenza, parainfluenza, herpes, coxsackie, ECHO, and adenovirus viruses should be determined. In this case, titration of the first and second sera should be carried out simultaneously. The list of serological studies performed according to indications can be expanded. For example, in areas endemic for tick-borne encephalitis, with the development of neurological diseases after vaccination carried out in the spring-summer period, the determination of antibodies to the tick-borne encephalitis virus is justified.
If a lumbar puncture is performed, it is necessary to conduct a virological study of the cerebrospinal fluid in order to isolate both vaccine viruses (when vaccinated with live vaccines) and viruses that are possible causative agents of intercurrent disease. The material should be delivered to the virology laboratory either frozen or at the temperature of melting ice. In the cells of the cerebrospinal fluid sediment obtained by centrifugation, indication of viral antigens in the immunofluorescence reaction is possible.
In case of serous meningitis that developed after mumps vaccination or suspected VAP, special attention should be paid to the indication of enteroviruses.
When making a clinical diagnosis of a generalized BCG infection, verification by bacteriological methods involves isolating a culture of the pathogen with subsequent proof of its belonging to Mycobacterium bovis BCG.
A separate group consists of complications that developed as a result of so-called software errors. The latter include: violation of the dose and method of administration of the drug, erroneous administration of another drug, non-compliance with the general rules of vaccination. As a rule, such violations are committed by medical workers, primarily nurses, who have not been trained in vaccine prevention. A distinctive feature of this kind of complications is their development in persons vaccinated in the same institution or by the same medical worker.
When treating a disease that arose in the post-vaccination period, the clinician and the pathologist in the case of death should be focused on the possibility of the development of a complex combined pathology during this period.
Prevention of post-vaccination complications. Vaccination of special groups
Reducing the number of contraindications to vaccination raises the question of developing rational tactics for vaccinating children with certain health conditions that are not a contraindication to vaccination. The designation of such children as a “risk group” is unjustified, since we are not talking about the risk of vaccination, but about choosing the most appropriate time and technique for its implementation, as well as methods for treating the underlying disease with achieving the most complete remission possible. The name “special or special groups” is more justified, requiring certain precautions when carrying out vaccinations.
Reactions to previous vaccine doses
Continued administration of the vaccine is contraindicated in children who develop a severe reaction or complication after receiving this drug.
Severe reactions include the following: temperature 40 C or higher; local reaction 8 cm in diameter or more.
Complications include: encephalopathy; convulsions; severe immediate reactions of the anaphylactic type (shock, Quincke's edema); hives; prolonged high-pitched scream; collaptoid states (hypotensive-hypodynamic reactions).
If the occurrence of these complications is associated with the administration of DTP vaccine, subsequent vaccination is carried out with DTP toxoid.
In rare cases of the occurrence of such reactions to ADS or ADS-M, completion of vaccination according to epidemiological indications can be carried out with the same vaccines against the background of the administration (one day before and 2 - 3 days after vaccination) of steroids (internal prednisolone 1.5 - 2 mg/kg/ day or another drug in an equivalent dose). The same method can be used when administering DPT to children who have given a pronounced reaction to the DTP vaccine.
Live vaccines (OPV, LCV, LPV) are administered to children with a reaction to DTP as usual.
If a child has had an anaphylactic reaction to antibiotics or culture substrate antigens contained in live vaccines (chicken egg white in influenza vaccines, as well as in foreign measles and mumps vaccines), subsequent administration of these and similar vaccines is contraindicated. In Russia, Japanese quail eggs are used for the production of LCV and LPV, so the presence of hypersensitivity to chicken egg protein is not a contraindication for their administration. Contraindications to revaccination with BCG and OPV are also specific complications that developed after the previous administration of the drug.
After completing the investigation of the case of PVO, the commission draws up an epidemiological investigation report in accordance with the guidelines for “Monitoring post-vaccination complications.”
Monitoring post-vaccination complications
Monitoring of post-vaccination complications is a system of continuous monitoring of the safety of medical immunobiological drugs (MIBPs) in the conditions of their practical use.
The purpose of monitoring is to obtain materials indicating the safety of MIBP and to improve the system of measures to prevent post-vaccination complications (PVO) after their use.
According to the WHO: “Identification of post-vaccination complications, followed by their investigation and action, increases public acceptance of immunization and improves health care. This, first of all, increases immunization coverage of the population, which leads to a decrease in morbidity. Even if the cause cannot be determined or the disease was caused by a vaccine, the fact that a case of post-vaccination complications is investigated by medical workers increases public confidence in vaccinations.”
Monitoring tasks include:
- oversight of MIBP safety;
- identification of post-vaccination complications after the use of domestic and imported MIBPs;
- determination of the nature and frequency of PVO for each drug;
- identification of factors contributing to the development of air defense, including demographic, climatic-geographical, socio-economic and environmental, as well as those determined by the individual characteristics of the vaccinated person.
Monitoring of post-vaccination complications is carried out at all levels of medical care of the population: district, city, regional, regional, republican. It applies to federal, municipal and private healthcare organizations, as well as citizens engaged in private medical practice if they have licenses for the relevant types of activities in the field of immunoprophylaxis.
N. I. Briko - Academician of the Russian Academy of Sciences, Professor, Doctor of Medical Sciences, Head of the Department of Epidemiology and Evidence-Based Medicine of the First Moscow State Medical University named after. THEM. Sechenov, President of NASKI.
Other news
Epidemiologist Nikolai Briko: the situation with coronavirus in Russia is quite difficult
The coronavirus pandemic has forced doctors and scientists to reassess the situation with infectious diseases in Russia. In particular, in recent years, the country has seen an increase in the incidence of meningococcal infection, which indicates the importance of implementing measures to prevent it. Academician of the Russian Academy of Sciences, Doctor of Medical Sciences, Professor Nikolai Briko spoke about which categories of citizens are most susceptible to meningococcal infection, the consequences of its combination with COVID-19, as well as the overall situation with coronavirus in the country.
Rospotrebnadzor warned about the danger of “chickenpox parties”
Some people believe that it is better to have chickenpox in childhood, because a child supposedly tolerates this disease more easily than an adult. Therefore, there are still parents who try to “plan” for the disease: they go to visit sick friends and organize “chickenpox parties,” Alexander Gorelov, deputy director for scientific work at the Central Research Institute of Epidemiology of Rospotrebnadzor, noted in an interview with Sputnik radio.
In the Nizhny Novgorod region, 1.5 million people were vaccinated against influenza
In the Nizhny Novgorod region, flu vaccination is being completed, the press service of the regional government reported. The vaccination campaign covered less than half of the region’s population—1.5 million people, including 388 thousand children and adolescents.
In Bashkiria they talked about the benefits of vaccination against pneumococcus
Does the pneumococcal vaccination make it easier to cope with coronavirus in case of complications? Important information was presented at a briefing on the results of the activities of the healthcare sector in Bashkiria in 2021. As Industry Minister Maxim Zabelin said, indeed, vaccination against pneumococcus reduces the incidence of complications from Covid and the development of bacterial pneumonia.
Rostec began supplying Russian flu vaccines abroad
In 2021, FORT exported vaccines to the Republic of Belarus, Moldova, Kyrgyzstan, Turkmenistan and Uzbekistan. In the overall structure of supplies, the largest volume (over 90%) fell on the quadrivalent vaccine Ultrix Quadri. In addition, foreign partners purchased the trivalent Ultrix vaccine.
Nacimbio launched an application about vaccinations
, which is managed by the Nacimbio holding of the Rostec State Corporation, launched the “Vaccinations - Personal Calendar” mobile application. It helps users create an individual vaccination schedule and reminds them about scheduled vaccinations. The Vaccinations app is available for free on Android and iOS mobile phones.
Covid-19 vaccines will be included in the preventive vaccination calendar
Prime Minister Mikhail Mishustin instructed to include vaccines against the new coronavirus infection in the calendar of preventive vaccinations for epidemic indications, taking into account priority. Previously, COVID-19 vaccines were included in the VED. Mishustin noted that changes to the order of the Ministry of Health “On approval of the National Calendar of Preventive Vaccinations and the Calendar of Preventive Vaccinations for Epidemic Indications” must be made before December 17, 2020.
Vaccination against coronavirus has begun in Crimea
“Vaccination begins today in Crimea. First of all, high-risk medical workers who work with patients with a new coronavirus infection will be vaccinated. In the future, the daily number of vaccinated people will depend on the volume of vaccine received,” wrote the head of the republic, Sergei Aksenov, on his page on the VKontakte social network.
Cases of dangerous measles continue to be recorded in the Volgograd region
In the Volgograd region in 2021, measles was laboratory confirmed. This was reported by the regional department of Rospotrebnadzor. It is known that a dangerous disease manifests itself in those who have not been vaccinated. This year, 1 case of measles was confirmed in the region.
Scientists will combine the Oxford vaccine with Sputnik V
Scientists from the University of Oxford and the Russian Gamaleya Research Center for Epidemiology and Epidemiology will join forces to work on a vaccine for COVID-19. They agreed to conduct clinical trials of a combination of two drugs - the British vaccine and one of the two components of the Russian Sputnik V, the Russian Direct Investment Fund and the Swedish-British pharmaceutical company AstraZeneca reported.